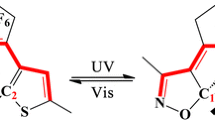
Abstract
A non-photochromic diarylethene 2o with an N-phenylaza-15-crown-5 was synthesized. When the nitrogen atom in the aza-crown ring was protonated, it became photochromic due to the prevention of a twisted intramolecular charge transfer (TICT). Although addition of a monovalent metal cation (Li+, Na+, K+, Rb+, Cs+, Cu+, Ag+) in acetonitrile could not stop the TICT so that it was not photochromic, the addition of a multivalent metal cation (Mg2+, Ca2+, Sr2+, Ba2+, Fe2+, Ni2+, Al3+, Sb5+) changed 2o to be photochromic due to the strong attraction of the lone pair on the nitrogen atom. In the presence of excess Cu2+, 2o was oxidized to be EPR-detectable 2o·+, which was thermally unstable as well as inert towards visible-light irradiation. However, 2o·+ was further oxidized to be fairly stable 2o2+ by the irradiation of 365-nm light in the presence of Cu2+. ESI–MS measurements strongly suggested the generation of 2o·+ by mixing 2o with Cu(ClO4)2 in acetonitrile, and the transformation of 2o·+ to 2o2+ by successive 365-nm light irradiation. Fe3+ similarly worked as the oxidant, but the two-step oxidation of 2o to 2o2+ occurred more easily.
Graphical abstract














Copyright 2011 American Chemical Society)






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Fritzsche, M. (1867). Note sur les carbures d’hydrogène solides, tirés du goudron de houille. Comptes Rendus, 69(1), 1035–1037.
Hirshberg, Y. (1950). Photochromié dans la série de la bianthrone. Comptes Rendus, 231(2), 903–904.
Brown, G. H. (1971). Techniques of chemistry (Vol. 3). Wiley-Interscience.
Dürr, H., & Bouas-Laurent, H. (2003). Photochromism: Molecules and systems. Elsevier.
Crano, J. C., & Gugglielmetti, R. J. (1999). Organic photochromic and thermochromic compounds (Vols. 1–2). Plenum Press.
Feringa, B., & Browne, W. R. (2011). Molecular switches (2nd ed., Vols. 1–2). Wiley-VCH.
Irie, M., Yokoyama, Y., & Seki, T. (2013). New frontiers in photochromism. Springer.
Tian, H., & Zhang, J. (2016). Photochromic materials. Wiley-VCH.
Yokoyama, Y., & Nakatani, K. (2017). Photon-working switches. Springer.
Miyasaka, H., Matsuda, K., Abe, J., & Kawai, T. (2020). Photosynergetic responses in molecules and molecular aggregates. Springer.
Irie, M. (2000). Diarylethenes for memories and switches. Chemical Reviews, 100(5), 1685–1716. https://doi.org/10.1021/cr980069d
Irie, M., Fukaminato, T., Matsuda, K., & Kobatake, S. (2014). Photochromism of diarylethene molecules and crystals: Memories, switches, and actuators. Chemical Reviews, 114(24), 12174–12277. https://doi.org/10.1021/cr500249p
Irie, M. (2021). Diarylethene molecular photoswitches: Concepts and functionalities. Wiley-VCH.
Wezenberg, S. J. (2022). Photoswitchable molecular tweezers: Isomerization to control substrate binding, and what about vice versa? Chemical Communications, 58(79), 11045–11058. https://doi.org/10.1039/D2CC04329G
Bouas-Laurent, H., & Dürr, H. (2001). Organic photochromism (IUPAC technical report). Pure and Applied Chemistry, 73(4), 639–665. https://doi.org/10.1351/pac200173040639
Yumoto, K., Irie, M., & Matsuda, K. (2008). Control of the photoreactivity of diarylethene derivatives by quaternarization of the pyridylethynyl group. Organic Letters, 10(10), 2051–2054. https://doi.org/10.1021/ol8005216
Mahvidi, S., Takeuchi, S., Kusumoto, S., Sato, H., Nakagawa, T., & Yokoyama, Y. (2016). Gated photochromic system of diarylethene with a photon-working key. Organic Letters, 18(19), 5042–5045. https://doi.org/10.1021/acs.orglett.6b02494
Hou, I.C.-Y., Berger, F., Narita, A., Müllen, K., & Hecht, S. (2020). Proton-gated ring-closure of a negative photochromic azulene-based diarylethene. Angewandte Chemie International Edition, 59(42), 18532–18536. https://doi.org/10.1002/anie.202007989
Liu, H.-H., & Chen, Y. (2010). Carbon dioxide and water as a key for unlocking photochromism of diarylethene derivative. Journal of Photochemistry and Photobiology, A: Chemistry, 215(1), 103–107. https://doi.org/10.1016/j.jphotochem.2010.08.002
Song, B., Li, H., Yang, L., Zhang, F., & Xiang, J. (2012). Acid/base gated photochromism of diarylethenes with quinoline derivatives. Chinese Journal of Chemistry, 30(7), 1393–1398. https://doi.org/10.1002/cjoc.201200128
Zhang, J., Tan, W., Meng, X., & Tian, H. (2009). Soft mimic gear-shift with a multi-stimulus modified diarylethene. Journal of Materials Chemistry, 19(32), 5726–5729. https://doi.org/10.1039/b908707a
Wu, Y., Chen, S., Yang, Y., Zhang, Q., Xie, Y., Tian, H., & Zhu, W. (2012). A novel gated photochromic reactivity controlled by complexation/dissociation with BF3. Chemical Communications, 48(4), 528–530. https://doi.org/10.1039/C1CC15824D
Weng, T., Zhang, K., Wu, B., Chen, X., Zou, Q., Zeng, T., & Zhu, L. (2019). Orthogonally incorporating dual-fluorescence control into gated photochromism for multifunctional molecular switching. Chemistry—A European Journal, 25(67), 15281–15287. https://doi.org/10.1002/chem.201903759
Hu, X. G., Li, X. L., Kim, H. K., Ahn, K.-H., & Yang, S. I. (2020). Gated photochromic reactivity of azadithiacrown-ether functionalized diarylethene. Dyes and Pigments, 172, 107869. https://doi.org/10.1016/j.dyepig.2019.107869
Hu, X. G., Li, X. L., Ahn, K.-H., & Yang, S. I. (2020). Synthesis and characterization of gated photochromic diarylethene functionalized with dipicolylamine. Dyes and Pigments, 176, 108202. https://doi.org/10.1016/j.dyepig.2020.108202
Li, Y., Chen, X., Weng, T., Yang, J., Zhao, C., Wu, B., Zhang, M., Zhu, L., & Zou, Q. (2020). A monomolecular platform with varying gated photochromism. RSC Advances, 10(69), 42194–42199. https://doi.org/10.1039/d0ra08214g
Poon, P.C.-T., Lam, W. H., & Yam, V.W.-W. (2011). Gated photochromism in triarylborane-containing dithienylethenes: A new approach to a “lock-unlock” system. Journal of the American Chemical Society, 133(49), 19622–19625. https://doi.org/10.1021/ja208097a
Irie, M., Miyatake, O., & Uchida, K. (1992). Blocked photochromism of diarylethenes. Journal of the American Chemical Society, 114(22), 8715–8716. https://doi.org/10.1021/ja00048a063
Irie, M., Miyatake, O., Uchida, K., & Eriguchi, T. (1994). Photochromic diarylethenes with intralocking arms. Journal of the American Chemical Society, 116(22), 9894–9900. https://doi.org/10.1021/ja00101a010
Liu, K., Wen, Y., Shi, T., Li, F., Zhao, Y., Huang, C., & Yi, T. (2014). DNA gated photochromism and fluorescent switch in a thiazole orange modified diarylethene. Chemical Communications, 50(65), 9141–9144. https://doi.org/10.1039/c4cc02783c
Mao, Y., Liu, K., Lv, G., Wen, Y., Zhu, X., Lan, H., & Yi, T. (2015). CB[8] gated photochromism of a diarylethene derivative containing thiazole orange groups. Chemical Communications, 51(30), 6667–6670. https://doi.org/10.1039/c5cc01390a
Ohsumi, M., Fukaminato, T., & Irie, M. (2005). Chemical control of the photochromic reactivity of diarylethene derivatives. Chemical Communications, 2005(31), 3921–3923. https://doi.org/10.1039/b506801k
Lemieux, V., & Branda, N. R. (2005). Reactivity-gated photochromism of 1,2-dithienylethenes for potential use in dosimetry applications. Organic Letters, 7(14), 2969–2972. https://doi.org/10.1021/ol050971p
Kühni, J., & Belser, P. (2007). Gated photochromism of 1,2-diarylethenes. Organic Letters, 9(10), 1915–1918. https://doi.org/10.1021/ol070487h
Li, X., Ma, Y., Wang, B., & Li, G. (2008). “Lock and key control” of photochromic reactivity by controlling the oxidation/reduction state. Organic Letters, 10(16), 3639–3642. https://doi.org/10.1021/ol8013655
Nourmohammadian, F., Wu, T., & Branda, N. R. (2011). A ‘chemically-gated’ photoresponsive compound as a visible detector for organophosphorus nerve agents. Chemical Communications, 47(39), 10954–10956. https://doi.org/10.1039/c1cc13685b
Song, B., Li, H., Yang, L., Zhao, C., Sai, H., Zhang, S., Zhang, F., & Xiang, J. (2012). Esterifiable/hydrolytic control of photochromism of diarylethenes with 8-hydroxyquinoline derivatives. Journal of Photochemistry and Photobiology, A: Chemistry, 241(1), 21–25. https://doi.org/10.1016/j.jphotochem.2012.05.005
Asadirad, A. M., Boutault, S., Emo, Z., & Branda, N. R. (2014). Controlling a polymer adhesive using light and a molecular switch. Journal of the American Chemical Society, 136(8), 3024–3027. https://doi.org/10.1021/ja500496n
Kida, J., Imato, K., Goseki, R., Aoki, D., Morimoto, M., & Otsuka, H. (2018). The photoregulation of a mechanochemical polymer scission. Nature Communications, 9, 3504. https://doi.org/10.1038/s41467-018-05996-7
Barber, R. W., McFadden, M. E., Hu, X., & Robb, M. J. (2019). Mechanochemically gated photoswitching: Expanding the scope of polymer mechanochromism. Synlett, 30(15), 1725–1732. https://doi.org/10.1055/s-0037-1611858
Irie, M., & Sayo, K. (1992). Solvent effects on the photochromic reactions of diarylethene derivatives. Journal of Physical Chemistry, 96(19), 7671–7674. https://doi.org/10.1021/j100198a035
Ohsumi, M., Hazama, M., Fukaminato, T., & Irie, M. (2008). Photocyclization reaction of a diarylmaleimide derivative in polar solvents. Chemical Communications, 2008(28), 3281–3283. https://doi.org/10.1039/B802780C
Kobatake, S., Terakawa, Y., & Imagawa, H. (2009). Solvent effect on photochromism of a dithienylperfluorocyclopentene having diethylamino group. Tetrahedron, 65(31), 6104–6108. https://doi.org/10.1016/j.tet.2009.05.053
de Silva, A. P., Gunaratne, H. Q. N., Gunnlaugsson, T., Huxley, A. J. M., McCoy, C. P., Rademacher, J. T., & Rice, T. E. (1997). Signaling recognition events with fluorescent sensors and switches. Chemical Reviews, 97(5), 1515–1566. https://doi.org/10.1021/cr960386p
Junk, P. C., & Steed, J. W. (1999). Crown ether chemistry of the alkaline earth nitrates. Journal of the Chemical Society, Dalton Transactions, 1999(3), 407–414. https://doi.org/10.1039/A807006G
Rounaghi, G. H., Khoshnood, R. S., & Zavvar, M. H. A. (2006). Study of complex formation between N-phenylaza-15-crown-5 with Mg2+, Ca2+, Ag+ and Cd2+ metal cations in some binary mixed aqueous and non-aqueous solvents using the conductometric method. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 54(3–4), 247–252. https://doi.org/10.1007/s10847-005-8380-7
Malval, J.-P., Gosse, I., Morand, J.-P., & Lapouyade, R. (2002). Photoswitching of cation complexation with a monoaza-crown dithienylethene photochrome. Journal of the American Chemical Society, 124(6), 904–905. https://doi.org/10.1021/ja0167203
Shannon, R. D. (1976). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst, A32(5), 751–767. https://doi.org/10.1107/S0567739476001551
Sumalekshmy, S., & Gopidas, K. R. (2005). Reaction of aromatic amines with Cu(ClO4)2 in acetonitrile as a facile route to amine radical cation generation. Chemical Physics Letters, 413(4–6), 294–299. https://doi.org/10.1016/j.cplett.2005.06.041
Kirchgessner, M., Sreenath, K., & Gopidas, K. R. (2006). Understanding reactivity patterns of the dialkylaniline radical cation. Journal of Organic Chemistry, 71(26), 9849–9852. https://doi.org/10.1021/jo061809i
Sreenath, K., Suneesh, C. V., Kumar, V. K. R., & Gopidas, K. R. (2008). Cu(II)-mediated generation of triarylamine radical cations and their dimerization. An easy route to tetraarylbenzidines. The Journal of Organic Chemistry, 73(8), 3245–3251. https://doi.org/10.1021/jo800349n
Sreenath, K., Thomas, T. G., & Gopidas, K. R. (2011). Cu(II) mediated generation and spectroscopic study of the tris(4-anisyl)amine radical cation and dication Unusually shielded chemical shifts in the dication. Organic Letters, 13(5), 1134–1137. https://doi.org/10.1021/ol103167m
Fenin, J. B., Mann, M., Meng, C. K., Wong, S. F., & Whitehouse, O. M. (1989). Electrospray ionization for mass spectrometry of large biomolecules. Science, 246(4926), 64–71. https://doi.org/10.1126/science.2675315
Bard, A. J. (1974). Encyclopedia of electrochemistry of the elements, (Vol. 2). Marcel Dekker.
Bard, A. J. (1982). Encyclopedia of electrochemistry of the elements (Vol. 9). Marcel Dekker.
Pavlishchuk, V. V., & Addison, A. W. (2000). Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25 °C. Inorganica Chimica Acta, 298(1), 97–102. https://doi.org/10.1016/S0020-1693(99)00407-7
Fox, M. A., & Hurst, J. R. (1984). Electrochemically induced pericyclic reactions. A radical anionic cyclization. Journal of the American Chemical Society, 106(24), 7626–7627. https://doi.org/10.1021/ja00336a055
Koshido, T., Kawai, T., & Yoshino, K. (1995). Optical and electrochemical properties of cis-1,2-dicyano-1,2-bis(2,4,5-trimethyl-3-thienyl)ethene. Journal of Physical Chemistry, 99(16), 6110–6114. https://doi.org/10.1021/j100016a055
Peters, A., & Branda, N. R. (2003). Electrochromism in photochromic dithienylcyclopentenes. Journal of the American Chemical Society, 125(12), 3404–3405. https://doi.org/10.1021/ja028764x
Peters, A., & Branda, N. R. (2003). Electrochemically induced ring-closing of photochromic 1,2-dithienylcyclopentenes. Chemical Communications, 2003(8), 954–955. https://doi.org/10.1039/B211378C
Gorodetsky, B., Samachetty, H. D., Donkers, R. L., Workentin, M. S., & Branda, N. R. (2004). Reductive electrochemical cyclization of a photochromic 1,2-dithienylcyclopentene dication. Angewandte Chemie International Edition, 43(21), 2812–2815. https://doi.org/10.1002/anie.200353029
Zhou, X.-H., Zhang, F.-S., Yuan, P., Sun, F., Pu, S.-Z., Zhao, F.-Q., & Tung, C.-H. (2004). Photoelectrochromic dithienylperfluorocyclopentene derivatives. Chemistry Letters, 33(8), 1006–1007. https://doi.org/10.1246/cl.2004.1006
Moriyama, Y., Matsuda, K., Tanifuji, N., Irie, S., & Irie, M. (2005). Electrochemical cyclization/cycloreversion reactions of diarylethenes. Organic Letters, 7(15), 3315–3318. https://doi.org/10.1021/ol051149o
Takaku, S., Nishimura, R., & Morimoto, M. (2023). A turn-on mode fluorescent diarylethene having an azacrown ether receptor: Metal-ion-gated enhancement of the photoreactivity and fluorescence. Dyes and Pigments, 216, 111354. https://doi.org/10.1016/j.dyepig.2023.111354
Balakit, A. A., Sert, Y., Çırak, Ç., Smith, K., Kariuki, B. M., & El-Hiti, G. A. (2017). Synthesis, vibrational spectra, and DFT simulations of 3-bromo-2-methyl-5-(4-nitrophenyl)thiophene. Journal of Applied Spectroscopy, 84(5), 888–899. https://doi.org/10.1007/s10812-017-0561-9
Kolmar, T., Büllmann, S. M., Sarter, C., Höfer, K., & Jäschke, A. (2021). Development of high-performance pyrimidine nucleoside and oligonucleotide diarylethene photoswitches. Angewandte Chemie International Edition, 60(15), 8164–8173. https://doi.org/10.1002/anie.202014878
Fukaminato, T., Hirose, T., Doi, T., Hazama, M., Matsuda, K., & Irie, M. (2014). Molecular design strategy toward diarylethenes that photoswitch with visible light. Journal of the American Chemical Society, 136(49), 17145–17154. https://doi.org/10.1021/ja5090749
Acknowledgements
This work was supported in part by JSPS KAKENHI Grant Number JP26107009 in Scientific Research on Innovative Areas “Photosynergetics.” The authors are grateful to the Instrumental Analysis Center of Yokohama National University for its support in the NMR and MS measurements. Thanks are also extended to Mr. Tsuyoshi Ito, Yokohama National University, for his support in the spectral measurements for the characterization of compounds. We are indebted to the Zeon Corp. for their generous donation of octafluorocyclopentene. Part of this study was presented at the 9th International Symposium on Photochromism (ISOP2019), September 2019, Paris.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takeguchi, A., Kikuchi, A., Ueno, K. et al. Ion valence-gated photochromism of an aza-crowned diarylethene. Photochem Photobiol Sci 23, 133–151 (2024). https://doi.org/10.1007/s43630-023-00508-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00508-z