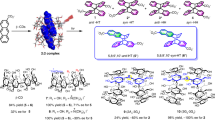
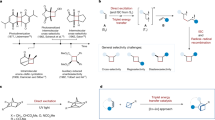
Abstract
Crossed [2 + 2] photocycloaddition is a specific case of intramolecular photocycloaddition reaction. Recently, the term “crossed [2 + 2] photocycloaddition” is interchangeably used to represent intermolecular [2 + 2] photocycloaddition reactions of two dissimilar double bonds/alkenes. To avoid confusion and to help researchers use the correct terminologies, this perspective clarifies the terminology used for different [2 + 2] photocycloaddition processes based on prior literature with the hope of establishing a standard for addressing the diverse set of photocycloaddition reactions that will be helpful to the chemical community.
Graphical abstract








Similar content being viewed by others
References
Turro, N. J., Ramamurthy, V., & Scaiano, J. C. (2010). Photochemistry of olefins. In Modern molecular photochemistry of organic molecules (p. 759). University Science Books
Poplata, S., Tröster, A., Zou, Y.-Q., & Bach, T. (2016). Recent advances in the synthesis of cyclobutanes by olefin [2 + 2] photocycloaddition reactions. Chemical Reviews, 116, 9748–9815. https://doi.org/10.1021/acs.chemrev.5b00723
Ramamurthy, V., & Sivaguru, J. (2016). Supramolecular photochemistry as a potential synthetic tool: Photocycloaddition. Chemical Reviews, 116, 9914–9993. https://doi.org/10.1021/acs.chemrev.6b00040
Corey, E. J., Bass, J. D., LeMahieu, R., & Mitra, R. B. (1964). A study of the photochemical reactions of 2-cyclohexenones with substituted olefins. Journal of the American Chemical Society, 86, 5570–5583. https://doi.org/10.1021/ja01078a034
Bauslaugh, P. G. (1970). Photochemical cycloaddition reactions of enones to alkenes; synthetic applications. Synthesis, 1970, 287–300.
Eaton, P. E. (1962). On the mechanism of the photodimerization of cyclopentenone. Journal of the American Chemical Society, 84, 2454–2455. https://doi.org/10.1021/ja00871a039
Hammond, G. S., Stout, C. A., & Lamola, A. A. (1964). Mechanisms of photochemical reactions in solution. XXV. The photodimerization of coumarin. Journal of the American Chemical Society, 86, 3103–3106. https://doi.org/10.1021/ja01069a026
Morrison, H. A., Curtis, H., & McDowell, T. (1966). Solvent effects on the photodimerization of coumarin. Journal of the American Chemical Society, 88, 5415–5419.
Muthuramu, K., & Ramamurthy, V. (1982). Photodimerization of coumarin in aqueous and micellar media. Journal of Organic Chemistry, 47, 3976–3979. https://doi.org/10.1021/jo00141a035
Moorthy, J. N., Venkatesan, K., & Weiss, R. G. (1992). Photodimerization of coumarins in solid cyclodextrin inclusion complexes. Journal of Organic Chemistry, 57, 3292–3297.
Blum, T. R., Miller, Z. D., Bates, D. M., Guzei, I. A., & Yoon, T. P. (2016). Enantioselective photochemistry through Lewis acid-catalyzed triplet energy transfer. Science, 354, 1391–1395. https://doi.org/10.1126/science.aai8228
Hörmann, F. M., Chung, T. S., Rodriguez, E., Jakob, M., & Bach, T. (2018). Evidence for triplet sensitization in the visible-light-induced [2 + 2] photocycloaddition of eniminium ions. Angewandte Chemie International Edition, 57, 827–831. https://doi.org/10.1002/anie.201710441
Ahuja, S., Raghunathan, R., Kumarasamy, E., Jockusch, S., & Sivaguru, J. (2018). Realizing the photoene reaction with alkenes under visible light irradiation and bypassing the favored [2 + 2]-photocycloaddition. Journal of the American Chemical Society, 140, 13185–13189. https://doi.org/10.1021/jacs.8b08100
Srinivasan, R., & Carlough, K. H. (1967). Mercury(3P1) photosensitized internal cycloaddition reactions in 1,4-, 1,5-, and 1,6-dienes. Journal of the American Chemical Society, 89, 4932–4936. https://doi.org/10.1021/ja00995a018
Liu, R. S. H., & Hammond, G. S. (1967). Photosensitized internal addition of dienes to olefins. Journal of the American Chemical Society, 89, 4936–4944. https://doi.org/10.1021/ja00995a019
Wiesner, K., Musil, V., & Wesner, K. J. (1968). Syntheses in the series of lycopodium alkaloids. IX. Two simple stereospecific syntheses of 12-epi-lycopodine. Tetrahedron Letters, 9, 5643–5646. https://doi.org/10.1016/S0040-4039(00)70741-6
Brown, M. (1965). The photocyclization of an acyclic bis-unsaturated carbonyl system. Chemical Communications. https://doi.org/10.1039/C19650000340
Kumarasamy, E., & Sivaguru, J. (2013). Light-induced stereospecific intramolecular [2 + 2]-cycloaddition of atropisomeric 3,4-dihydro-2-pyridones. Chemical Communications, 49, 4346–4348. https://doi.org/10.1039/C2CC37123E
Gravatt, C. S., Melecio-Zambrano, L., & Yoon, T. P. (2021). Olefin-supported cationic copper catalysts for photochemical synthesis of structurally complex cyclobutanes. Angewandte Chemie International Edition, 60, 3989–3993. https://doi.org/10.1002/anie.202013067
Srinivasan, R. (1963). Mercury photosensitized isomerization of 1,5-cycloöctadiene to tricyclo [3.3.0.02,6] octane. Journal of the American Chemical Society, 85, 819–820. https://doi.org/10.1021/ja00889a039
Tamura, Y., Kita, Y., Ishibashi, H., & Ikeda, M. (1971). Intramolecular photocycloaddition of 3-allyloxy- and 3-allylamino-cyclohex-2-enones: Formation of oxa- and aza-bicyclo[2,1,1]hexanes. Journal of the Chemical Society, Chemical Communications. https://doi.org/10.1039/C29710001167
Iyer, A., Jockusch, S., & Sivaguru, J. (2014). Dictating photoreactivity through restricted bond rotations: Cross-photoaddition of atropisomeric acrylimide derivatives under UV/visible-light irradiation. Journal of Physical Chemistry A, 118, 10596–10602. https://doi.org/10.1021/jp505678b
Zhao, J., Brosmer, J. L., Tang, Q., Yang, Z., Houk, K. N., Diaconescu, P. L., & Kwon, O. (2017). Intramolecular crossed [2+2] photocycloaddition through visible light-induced energy transfer. Journal of the American Chemical Society, 139, 9807–9810. https://doi.org/10.1021/jacs.7b05277
Bach, T., Bergmann, H., & Harms, K. (2000). Enantioselective intramolecular [2 + 2]-photocycloaddition reactions in solution. Angewandte Chemie International Edition, 39, 2302–2304. https://doi.org/10.1002/1521-3773(20000703)39:13%3c2302::AID-ANIE2302%3e3.0.CO;2-6
Rigotti, T., Schwinger, D. P., Graßl, R., Jandl, C., & Bach, T. (2022). Enantioselective crossed intramolecular [2+2] photocycloaddition reactions mediated by a chiral chelating Lewis acid. Chemical Science, 13, 2378–2384. https://doi.org/10.1039/D2SC00113F
Gleiter, R., & Sander, W. (1985). Light-induced [2 + 2] cycloaddition reactions of nonconjugated dienes—the effect of through-bond interaction. Angewandte Chemie International Edition, 24, 566–568. https://doi.org/10.1002/anie.198505661
Bradley, S. A., Bresnan, B. J., Draper, S. M., Geraghty, N. W. A., Jeffares, M., McCabe, T., McMurry, T. B. H., & O’Brien, J. E. (2011). Photochemical [2 + 2] cycloaddition reactions of 6-alkenyl-3-phenylcyclohex-2-en-1-ones: Using biradical conformation control to account for exceptions to the “rule of five.” Organic & Biomolecular Chemistry, 9, 2959–2968. https://doi.org/10.1039/C0OB01131B
Dilling, W. L. (1966). Intramolecular photochemical cycloaddition of nonconjugated olefins. Chemical Reviews, 66, 373–393. https://doi.org/10.1021/cr60242a002
Alder, A., Bühler, N., & Bellus, D. (1982). A note on intramolecular photochemical cycloaddition of N-substituted dimethacrylimides. Helvetica Chimica Acta, 65, 2405–2412. https://doi.org/10.1002/hlca.19820650805
Ahuja, S., Iyer, A., Kandappa, S. K., & Sivaguru, J. (2019). Photo-auxiliary approach to control excited state reactivity: Cross [2+2]-photocycloaddition of oxazolidinone based hydrazides. Journal of Photochemistry and Photobiology Sciences: A Chemistry, 382, 111883. https://doi.org/10.1016/j.jphotochem.2019.111883
Turnbull, A. G., & Hull, H. S. (1968). A thermodynamic study of the dimerization of cyclopentadiene. Australian Journal of Chemistry, 21, 1789–1797.
Krupka, J. (2015). Kinetics of Diels–Alder reactions between 1,3-cyclopentadiene and isoprene. Reaction Kinetics, Mechanisms and Catalysis, 116, 315–326. https://doi.org/10.1007/s11144-015-0913-5
Xu, R., Jocz, J. N., Wiest, L. K., Sarngadharan, S. C., Milina, M., Coleman, J. S., Iaccino, L. L., Pollet, P., Sievers, C., & Liotta, C. L. (2019). Cyclopentadiene dimerization kinetics in the presence of c5 alkenes and alkadienes. Industrial and Engineering Chemistry Research, 58, 22516–22525. https://doi.org/10.1021/acs.iecr.9b04018
Griffin, G., & Heep, U. (1970). The [2 + 2] photocycloadditions of indene and 2-and 3-chloroindenes. Journal of Organic Chemistry, 35, 4222–4224. https://doi.org/10.1021/jo00837a614
Xie, X., Pan, H., Zhou, T.-P., Han, M.-Y., Wang, L., Geng, X., Ma, Y., Liao, R.-Z., Wang, Z.-M., Yang, J., & Li, P. (2021). Ortho-ethynyl group assisted regioselective and diastereoselective [2 + 2] cross-photocycloaddition of alkenes under photocatalyst-, additive-, and solvent-free conditions. Organic Chemistry Frontiers, 8, 5872–5887. https://doi.org/10.1039/D1QO01017D
Acknowledgements
The authors thank the US National Science foundation (CHE-1955524 for JS and CHE-1807729 for VR), the German Science Foundation (TB) and the European Research Council (TB) for funding their respective research programs in which various [2+2] photocycloaddition strategies have been investigated.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sivaguru, J., Bach, T. & Ramamurthy, V. Keeping the name clean: [2 + 2] photocycloaddition. Photochem Photobiol Sci 21, 1333–1340 (2022). https://doi.org/10.1007/s43630-022-00239-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00239-7