Abstract
Due to a lack of organs, cardiac support systems are being implanted in patients with severe congestive heart failure. One of the solutions to overcome complications such as inflow obstruction or pump thrombosis, which may occur in the case of ventricular assist devices, is to modify the surface of cannulas for the controlled blood clotting process. The results obtained up till now for developed surface coatings clearly show the influence of topographical and mechanical parameters of the coatings on cell viability and protein adsorption mechanism. The new coatings should enable the controlled growth of scar tissue, resulting in the limitation of thromboembolic events, and the reduction of cystic tissue growth into the flow lumen. The aim of this study is to evaluate the correlation between surface topography parameters on the susceptibility of cells to grow and adhere to the substrate as a solution with potential for use in MCS (mechanical circulatory support) devices. Research on surfaces used in MCS devices and on inflow cannulas has been carried out for many years, while the novelty of the present solution makes it a milestone within that type of application simultaneously allowing for appropriate selection of process parameters. Surface modification of titanium alloy Ti6Al7Nb was carried out using vacuum powder sintering of CP-Ti (commercially pure titanium) powder with two morphologies (regular spheres and irregular grains). The characterization of coatings obtained with the proposed method and the influence of measured topographic parameters (applying scanning electron microscopy, contact angle measurement and contact profilometry) on the cytotoxicity and susceptibility to protein adsorption were presented. Advanced albumin adsorption studies have fully confirmed the dependence of surface complexity on protein adsorption. The obtained results show a high potential of the produced coatings toward enabling permanent integration at the implant with the soft tissue.
Similar content being viewed by others
1 Introduction
Heart failure is a disease that has recently affected an increasing number of patients. According to the current data, around 1 million people in Poland are living with a diagnosis of heart failure and over the next 20 years, this number will rise by 25% [1]. This disease causes high mortality among patients over 55 years of age (4-year survival is predicted in only about 50% of patients) [2]. Annually, heart failure is a direct cause of over 60,000 deaths despite the use of optimal pharmacological treatment and cardiac resynchronisation therapy. According to the recommendations of the European Society of Cardiology, the treatment of choice in this group of patients is heart transplantation. However, the intensity of heart transplantation procedures has remained stable for several years. In Europe, a total of around 600 heart transplantations are performed annually, according to the data recorded in Eurotransplant [3]. The number of patients with severe, pharmacologically refractory heart failure who qualified for heart transplantation has been increasing for several years and amounts to around 1200 patients per year in recent years. The large disproportion between the number of patients waiting for heart transplantation and the number of procedures performed, as well as the length of the waiting time for a heart transplant (the average waiting time for this surgery is 14 months), results in the high mortality rate of 22% in this group of patients [4]. Complementing optimal pharmacological treatment with mechanical heart support is a bridge to transplantation [5]. The introduction of centrifugal heart pumps to clinical use is a documented method of treatment used in severe heart failure—class I B medical recommendations of the European Society of Cardiology [6].
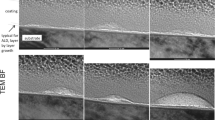
The importance of cardiac assist therapy cannot be underestimated, as despite the ever-evolving technology and new device constructions being developed, numerous complications remain [7]. In the 1980s, Frazer et al. demonstrated the dependence of surface topography on the occurrence of thromboembolic events in the therapy of mechanical circulatory support (MCS) [8]. The results of their research confirmed the validity of the use of textured surfaces in contact with blood. In the 90 s, the results of their research based on clinical data confirmed the validity of the use of textured surfaces for contact with blood in cardiac assist devices [9]. According to the literature surface texture may be determined as the local deviation of a surface from a perfectly flat plane. In general surface texture is defined in terms of its roughness, waviness and form which may be described as high degree of complexity characterized by high roughness and/or porosity [10]. Significant changes in trends concerning the use of long-term heart support have been observed for several years. Isolated left ventricular assist device (LVAD) is taking over the dominant role and the number of applications of heart support with implantable centrifugal pumps is growing particularly rapidly [11]. More and more new solutions of MCS devices are still being introduced to clinics, increasing the safety and comfort of the patient [12]. The changes mainly concern the selection of the most appropriate construction material and mechanics, considering various ways of suspending the rotor in the pump housing [13]. When the implant material is only tolerated by the body, there is still a risk of long-term complications [14]. Therefore, in these cases, it may be more advantageous to develop biofunctional coatings [15]. Such coatings may allow to restore the native function of the tissue by interacting with the bioactive coating that induces regenerative processes and repair responses [16]. In the case of centrifugal pumps, based on previous clinical experience, the potential of using textured surfaces was observed not only to reduce thromboembolic events, but also to achieve a stable contact bond at the implant-tissue interface. Najjar et al. [17] have pointed out that the most common complications after implantation of currently used 3rd generation ventricular assist devices (VAD), as exemplified by the HeartWare pump, are thromboembolic events and pathological hypertrophy of scar tissue into the inflow lumen. Thus, they indicate that the application of surface modification on the outer surface of the inflow cannula allows the inhibition of thrombotic processes and the formation of proper scar tissue. Najjar et al. [17] presented a comparative summary of the peri-implant tissue reactions to non-modified and modified surfaces of the inflow cannula. The main idea of this innovative solution is to allow controlled stimulation of the surrounding tissues in the area of the myocardial apex in order to provide tissue adherence and to cover only a specific area of the metallic element while preventing tissue overgrowth into the cannula’s lumen. Figure 1 shows the authors’ very early and not yet published own experiences with the use of the RELIGA HEART ROT pump after surface modification presented in this paper during preclinical trials. The experiments were planned and carried out based on the PN-EN ISO 10993 standard and with the consent of the ethical committee of the Medical University of Silesia in Katowice. Figure 1a presents the principle and position of the pump after implantation. The RELIGA HEART ROT pump with a close-up on the modified inflow cannula is presented respectively in Fig. 1b and c. Figure 1d presents the formation of proper scar tissue during preclinical trials around the inflow cannula, with no signs of pathological tissue overgrowth into the lumen of the flow. The authors' own clinical experience in comparison with the results of other cardiac centres in the world conducting research in the field of textured coatings for use in MCS pumps allows us to conclude about their potential to reduce thromboembolic events.
Polish centrifugal blood pump of the RELIGA HEART ROT dedicated to cardiac support system: a principle of implantation of a pump in the heart, b pump of the RELIGA HEART ROT appearance, c close-up on a modified inflow cannula of RELIGA HEART ROT, d peri-implant tissue positive reaction around the inflow cannula with the surface modification
One of the consequences of getting textured surfaces in direct contact with blood is that these surfaces rapidly clot after the product is placed into the body [18]. While this may appear to be a negative scenario, the concept behind this design is that while clots form quickly on these surfaces, they adhere tightly and do not appear to embolize into the bloodstream in a clinically significant way [19]. Over time, there is an additional interaction of blood cells, similar to an inflammatory reaction [20]. A heterogeneous surface containing platelets, monocytes, macrophages, giant body cells, lymphocytes and pluripotent blood cells is deposited [21]. It has been postulated that pluripotent hematopoietic cells differentiate into fibroblasts, myofibroblasts and in some cases endothelial cells, which cause the surface to become populated [22]. In contrast to the inflammatory reaction associated with the textured surfaces of cardiovascular biomaterials, cells that populate this textured surface probably come entirely from flowing blood [23]. Properly running healing processes result in the proliferation of fibroblastic cells and the final reconstruction of peri-implant tissues, thus inhibiting the inflammatory processes and inactivating the blood [24].
As emphasized earlier, the main complications that may occur in the case of ventricular assist devices with well-polished cannula are inflow obstruction and pump thrombosis [25]. The tissue overgrowth may cause adverse flow turbulences or suction events, disrupting the proper operation of the device [26]. That is why the aim of this study is an evaluation of the correlation between surface topography parameters on the susceptibility of cells to grow and adhere as a solution with potential for use in MCS devices. Proper selection of topographic parameters may stimulate the surrounding tissues to form a controlled scar ensuring stable apical placement and no overgrowth into the cannula’s lumen.
2 Materials and methods
2.1 Surface modification
The surface modification was carried out using the vacuum powder sintering (VPS) technique of commercially pure titanium powder CP-Ti (Commercially Pure Titanium) on Ti6Al7Nb substrate. The sintering process was carried out in the Biovac España S.A company, specializing in advanced surface treatment of medical devices and coating of surgical implants. The applied process parameters are covered by the secret of know-how due to the patent potential, but they do not constitute the considerations presented in the paper. The sintering process was carried out with the use of titanium powders with two types of grains—in the form of regular spheres (indicated as S) and irregular grains (indicated as I) in three gradations, taking into account the diameters of microspheres and the size of angular grains, i.e. S1, S2, S3 for regular spheres and I1, I2 and I3 for irregular grains. Thus, the numerical designation (1–3) denotes the modifications obtained with the use of powders with a higher gradation in the range of 90–250 µm, which results in an increase in surface roughness. The first attempts to apply VPS technology were promising, including the regularity of the surface and the obtained spaces between grains, which can stimulate cell growth. According to the information available in the literature, an optimal porosity of bioceramics in the range of 50 to 250 μm allows the ingrowth of mineralised and vascularised tissue [27]. Fibroblasts, however, tend to colonise smoother surfaces [28]. In this study, powders in the range of 90–250 μm were used. One of the assumptions of the proposed surface modification was to obtain a coating with a high roughness coefficient and complex morphology with a thickness of more than 200 µm. The tests involved flat cylindrical samples initially with a diameter of D = Ø14 ± 0.02 mm and a height h = 3 ± 0.02 mm. In the first step of materials testing, specimens were characterized with two grain types and three sizes using scanning electron microscopy (SEM), contact angle measurement and contact profilometry. Then, the biological response was analysed in terms of cytotoxicity and protein adsorption. The number of samples used each time was at least 5 according to the statistical principles.
2.2 Material structure and microstructure analysis
2.2.1 Surface analysis
In the case of digital microscopy, the Keyence VHX-5000 (Keyence Co., Osaka, Japan) device was used. The tests were carried out with the use of 500 × and 1000 × magnification and 3D stitching module. As far as scanning microscopy is concerned, SEM/Quanta/FEG 250/FE (FEI Co., Hillsboro, USA) was used. The observations were carried out in high vacuum conditions with a maximum value of 3.5 × 10–4 Pa using an ETD detector (Everhart–Thornley Detector) with an accelerating voltage of 5 kV.
The contact angle of the samples was tested by optical goniometry on the Möller-Wedel Optical instrument (Möller–Wedel Optical GmbH, Wedel, Germany) at room temperature (approx. 20 °C). Distilled water with a measuring droplet volume of 1.5 μl was used to moisten the surface. Before each measurement, the samples were cleaned and dried from residual water by compressed air.
2.2.2 Cross-sectional analysis
Prior to cross-sectional analysis, samples were cut using a Stuers Secotom-15 machine (Struers ApS, Hovedstaden, Denmark) with a 50A20 alumina wheel and then mounted with the use of Struers CitoPress-20 (Struers ApS, Hovedstaden, Denmark). The coating thickness and the degree of porosity were measured with the use of digital microscopy (Keyence VHX-5000). The measurement of porosity for each coating was carried out by preparing 3 cross-sections, ensuring liquid cooling to limit the occurrence of thermal material transformations. Then, 5 measurements were made for each cross-section. To estimate the maximum thickness of the coatings, a trend line was drawn for the highest peaks, then the height relative to the baseline of the substrate was averaged. Based on material and geotechnical engineering including the void ratio formula, a 2D porosity formula (1) has been proposed to determine the cross-sectional percentage of voids of the produced coating [29]. The Keyence image analysis module allowed porosity assessment determining the percentage ratio of empty spaces within the coating relative to the solid material:
where p – porosity [%]; \({S}_{p}\)– total surface area of the coating [µm2]; \({S}_{s}\) – surface area of the metal solid [µm2].
2.2.3 Surface roughness analysis
The surface roughness of the samples was assessed using both contact profilometry and optical profilometry in comparison to the samples, not subjected to surface modification in accordance with applicable standards—ISO 21920–2:2021. In the case of contact profilometry, a MAHR XR1 (Mahr GmbH, Göttingen, Germany) semi-automatic device was used, equipped with a BFW A 10-45-2/90º measuring arm. The surface topography was assessed based on the Ra and Rz parameters, following the statistical principles. In the case of optical profilometry, the Keyence VR-5000 (Keyence Co., Osaka, Japan) 3D optical profilograph was used and the attention was focused mainly on the Sa parameter and the qualitative topographic assessment. The Sa parameter is an extension of the Ra profile parameter in the context of surfaces. This parameter determines the absolute value of points in space as the difference in height with respect to the arithmetic mean value. In accordance with the recommendations of ISO 4288 and ISO 3274 and due to the diameter of the samples, the measured section length (Lt) was 12.5 mm, and the sampling section (Ls) was 2.5 mm for contact profilometry. However the surface area for the determination of the Sa parameter was an area of 9 mm2.
2.2.4 Structural analysis
The study allowed the evaluation of the surface chemical composition as a result of the technological process. For this purpose, the Quanta 250 FEG (FEI Co., Hillsboro, USA) high-resolution environmental scanning electron microscope was used, equipped with EDS / Apollo 10 / EDAX (Ametek) energy-dispersive X-ray spectroscopy. The test was carried out at 1000 × magnification under high vacuum conditions for an accelerating voltage of 10 kV.
2.2.5 Phase composition analysis
Analysis of the phase composition was performed to identify the crystalline phases present on the sample surface. Moreover, the crystallite sizes, unit cell parameters and sizes based on these parameters were also assessed. The study included the use of the Bruker D8 Discover system (Bruker Co., Billerica, USA), which allows for point analysis with a filtered X-ray beam and an automatic collection of X-ray spectra from any sequence of measurement points. The device was equipped with a cobalt anode, the radiation wavelength was CoKα = 1.79 Å, and the X-ray spectrum was recorded using a LynxEye strip detector. The measured angular range was from 40 to 80º and the step size was 0.01º.
2.3 Micromechanical testing
Micro/nano-indentation tests were carried out on the NHT-3 nano-hardness tester manufactured by Anton Paar (Anton Paar GmbH, Graz, Austria). Micromechanical properties were determined from the deformation of the material by indenting the sample with a Berkovich indenter for the initial load range of 50 mN–5 N. However, target measurements were performed for a 1 N maximum load on the basis of initial tests and the sensitivity analysis of the indentation force with respect to hardness and Young’s modulus. The values of the loading force and the penetration depth of the indenter blade were recorded continuously throughout the cycle (loading and unloading). Properties such as hardness, Young’s modulus, creep time, and fracture toughness were determined from the plotted load vs. displacement curve. By applying minimal indenter loading forces, it was possible to perform measurements at depths of 10 ± 1 µm, which is particularly important when testing coatings, since the influence of the substrate on the determined properties should be eliminated [30].
2.4 Cytotoxicity
In the next step, cytotoxicity studies were performed. Tests done in accordance with the guidelines of biocompatibility testing allowed the evaluation of the sintering process parameters, including grain shapes and sizes, and their influence on fibroblasts necrosis. The results were related to the control group, which was an unmodified Ti6Al7Nb alloy with confirmed biocompatibility [31]. A cytotoxicity test was performed by the direct method according to ISO 10993-5 on fibroblasts (L929 ATCC). The supplemented PromoCell Fibroblast Growth Medium 2 enriched with antibiotics (SIGMA ALDRICH Antybiotic Antymicotic Solution) was used as the medium. Cell viability was assessed after 24-h incubation using fluorescence method with the Carl Zeiss Exciter 5 confocal scanning microscope (Carl Zeiss, Jena, Germany). FDA (fluorescein tetraacetate) and PI (propidium iodide) were used for cell staining. Fluorescein accumulates in the cytoplasm of living cells and gives a green signal. Propidium iodide, however, stains the nucleus of necrotic cells in red.
2.5 Protein adsorption
Protein adsorption on the surface of the modified titanium alloy plays a key role in the colonization of an implant by cells, indirectly influencing increased biocompatibility. As part of the research, a quantitative analysis of the mass of the adsorbed protein (albumin) was performed with the use of human whole blood (B-RH +) and platelet-leukocyte concentrate. Protein quantitation is most commonly performed using colorimetric assays. Typical methods for the colorimetric determination of protein concentration in solution include the Coomassie blue G-250 dye–binding assay [32], the biuret method [33], the Lowry method [34], the bicinchoninic acid (BCA) assay [35], and the colloidal gold protein assay [36]. The most common protein assay reagents involve either protein–dye binding chemistry (Coomassie/Bradford) or protein–copper chelation chemistry (biuret/BCA). The tests included modified samples with extreme roughness, i.e. S1 and S3 as well as I1 and I3. After placing the samples in multiwell vessels, they were subjected to static incubation, respectively, in blood and concentrate for 60 min, but also with re-incubation for another 60 min with an intermediate wash step in a phosphate-buffered saline. Smooth samples in the original unmodified state were used as a control (Ref). After incubation, the samples were rinsed twice with PBS solution, and then their surfaces were treated with 1% Sodium Dodecyl Sulfate and left on a shaker for 1 h. The collected supernatant was prepared for tests with the use of Invitrogen Qubit® reagents according to the manufacturer’s protocol. Evaluation of the content of suspended albumin in the supernatant was performed using a Qubit® 2.0 calibrated fluorometer (Thermo Fisher Scientific, Waltham, USA). The study was extended with SEM microscopic observations allowing for the analysis of the method and location of cell adhesion.
3 Results
3.1 Surface structure analysis
Based on the surface analysis, a high diversity in the morphology and surface topography for the S and I modifications was observed—Fig. 2. The S coatings were characterized by the presence of a regular structure with rounded shapes being a result of the morphology of the powders used for its production. In the case of I coatings, numerous irregular elevations, craters and fractures were present revealing the substrate in the digital microscopy images (Fig. 2b). Based on the obtained microscopic images of the surface topography, a computer image analysis was carried out—Fig. 2e and f. The software converted the data into a 3D image using triangulation based on changes in the refraction bands that are caused by height variation. The results obtained with the 3D mapping confirmed the layered morphology of the developed coating. The software makes it impossible to normalize the Z scale determining peak heights, that is why the presented images have different colour scales in Fig. 2e and f. Nevertheless, in all cases, the images were taken with the same magnification and the peaks with the greatest height, marked in red, are presented in relation to the lowest layer possible to visualize by microscope, marked in blue.
Microscopic analysis of materials with modified surface using digital microscopy techniques: a surface with spherical structure, b surface with irregular structure; using scanning electron microscopy techniques: c surface with spherical structure, d surface with irregular structure, using 3D contour mapping on example of S1 e and S3 f with Ra roughness of 21 µm and 38 µm, respectively, for 1000 × magnification
Results of the wetting angle assessment are shown in Fig. 3c. Changes in the contact angle during 60 s for each measurement were determined basing on the image analysis. All samples after modification with the vacuum powder sintering method for the proposed powders are characterized by a contact angle of θ ≈ 103° ± 4. The highest contact angles are observed for samples S2 (107°) and I2 (106°). The lowest values of contact angle were recorded for samples S1 and I1, with values of θS1 = 102° and θI1 = 99°, respectively.
3.2 Cross-section analysis
The use of digital microscopy and image analysis software allowed the cross-sectional assessment of the coatings, both with spherical and irregular structures. The thicknesses of the coatings were determined for modification S in the range of h = 265–526 µm and for modification I in the range of h = 202–735 µm. The performed sintering process increased the height h of the samples in the range of 14.2–14.8 ± 0.01 mm depending on the powders used, while the diameter remained unchanged. As far as porosity is considered, an increase was observed along with the grain size of the powders for proposed modifications—Fig. 4. Modification with the use of spherical powders was characterized by higher porosity, ranging from 41 to 47%. In the case of modification with the use of irregular powders, the porosity reached the values of 29–37%.
3.3 Surface roughness analysis
Based on the roughness tests carried out by contact profilometry, an increase in the Ra and Rz parameters was noted together with the increase in the size of the powder grains used during the modification. The results obtained by contact profilometry for the Ra and Rz parameters are presented in Fig. 3a. Surface modification carried out with the use of spherical powders was characterized by the roughness values in the range of Ra = 21–38 µm and Rz = 129–209 µm. In the case of modifications for the powders with irregular crystals, the roughness was in the range of Ra = 22–48 µm and Rz = 131–274 µm. Similar dependence regarding the increase in the degree of roughness in relation to the size of the powder particles was noted by optical profilometry. The value of the surface roughness Sa parameter was assessed, which for the spherical modification was in the range of 28–58 µm, and for the irregular one was in the range of 25–56 µm (Fig. 3b).
3.4 Chemical microstructure and phase composition analysis
Based on the EDS analysis of chemical composition (Fig. 5a), the presence of titanium was observed for all types of coatings (peaks at 4.6 keV for Ti Kα, 0.4 keV for Ti Lα and 4.9 keV for Ti Kβ). Moreover, the presence of the peaks of Al and Nb alloying elements was observed for the energies of 1.5 keV and 2.2 keV, respectively, which, due to the high degree of porosity, reflect from the substrate of the initial Ti6Al7Nb material. The X-ray diffraction patterns of samples modified with both spherical and irregular powders are shown in Fig. 5b. The titanium α phase was identified as the main crystalline phase present in all samples. The peak at 2θ of about 47° can be attributed to Ti (101) crystallographic orientation, peaks at 2θ of 41°, 63° and 76° may be assigned to Ti (100), Ti (102) and Ti (110), respectively. The phase variation of Ti (101) in terms of crystallite position and size for all samples is shown in Table 1. In the case of S1, S2 and I2 modifications, the presence of a peak with low intensity can be attributed to the presence of the Ti-Nb-Al (012) phase.
3.5 Micromechanical testing
At the quality control stage of the sintering process, a peel test was carried out in accordance with the ASTM F1147-05 (2017) standard, and all obtained coatings were characterized by a strength of above 34.5 MPa. During indentation each of the samples showed a high tendency for microcracking. Figure 6 shows the summary of micromechanical properties of the obtained coatings, distinguishing the indentation microhardness (HiT) and the indent modulus of elasticity (EiT). Due to high porosity, the coatings are characterized by low coherence, which may justify the occurrence of microcracks. Samples modified with the use of irregular powders are characterized by lower values of both HiT and EiT than the S-series. Coatings obtained with the use of spherical powders are characterized by higher values of EiT ranging from 60 to 83 GPa.
3.6 Cytotoxicity
The most representative images taken with a Carl Zeiss LSM Exciter 5 microscope after FDA and PI staining are presented in Fig. 7a and Fig. 7b. The use of dyes allowed distinguishing necrotic cells (stained red) from live cells (stained green). Detailed analysis of the obtained results was carried out with the use of the colocalization function of signals coming from the FDA and PI. This function allows the separation of signals and makes it possible to determine the intensity of fluorescence, which depends on the number of fluorochrome particles associated with a specific cell structure.
Cytotoxicity assessment: a, b presents the fluorescence analysis by confocal microscopy of two representative surfaces, respectively, S1 and I1 in the context of the number of viable cells during 24 h test, c presents summary of the number of living and necrotic cells, d) presents cell survival rate [%] on the surfaces obtained with the use of all powders
The dependence of the number of necrotic cells on living cells for each modification is presented in Fig. 7c. The red line is a trend line below which all of the samples show no cytotoxic effects.
The cell survival graph based on the fluorescent images and colocalization analysis is presented in Fig. 7d. According to ISO 10993: “A decrease in cell viability by more than 30% is considered a cytotoxic effect.” In this case, no cytotoxic effect was observed for all modifications, and the survival rate ranged from 85 to 96% after 24 h of incubation. The highest survival rate of 96% was observed for the modification marked as I1.
3.7 Protein adsorption
Figure 8 demonstrates a study on protein adsorption to the surface of coatings in different media, i.e. the whole blood and platelet-leukocyte concentrate. Based on this analysis, the positive effect of coatings on the susceptibility of albumin to surface attachment in relation to the control sample was observed. In the case of single (60 min) incubation in blood, modification S3 and I3 were characterized by the highest albumin concentration, 2495 ng/µl and 1354 ng/µl, respectively. However, modified samples analysed after single incubation in platelet-leukocyte concentrate reached comparable values of 448 ng/µl. In the case of double incubation (2 × 60 min), samples of all modifications reached the albumin concentration in the range of 453–541 ng/µl for the whole blood, and 467 ng/µl for the platelet-leukocyte concentrate (Fig. 8a). The control sample (Ref) reached the values of 131 ng/µl and 119 ng/µl, respectively. In the case of whole blood, a similar level ≈ 477 ng/µl of albumin was observed for samples S3 and I3. Sample S3 also showed the greatest susceptibility to albumin binding from the concentrate reaching the value of 530 ng/µl. The lowest level of albumin was detected for modification I1, reaching the value of 404 ng/µl. After double incubation (2 × 60 min) in the whole blood, the highest susceptibility to albumin attachment was for sample S1 (540 ng/µl), whereas the lowest affinity of surface to protein was found for I1 (453 ng/µl). On the basis of the scanning microscopy images, it was possible to visualize the morphotic parts of blood, including erythrocytes and thrombocytes within the developed coating. The study showed the locations most susceptible to attachment/implantation of blood cells. The study showed that cells most willingly settle in the corners of structures formed after powders sintering between their grains—Fig. 8b and c. Concerning the cell attachment is not possible to evaluate using only SEM technique. It would be necessary to consider dynamic test of the cell detachment analysis. However obtained results confirm the validity of developing coatings with a high degree of differentiation or porosity in order to provide integration at the implant-tissue interface.
4 Discussion
The results obtained for developed surface coatings clearly show the influence of topographical and mechanical parameters on cell viability and protein adsorption. The research reported in the work is fundamental with an eminently applicable intended target [9] and clinical confirmation [17]. Cannula designs [37] and surface-developed coatings [38] are of interest to companies leading in the development of centrifugal heart assist systems. The issue presented in the manuscript concerns the impact of the applied modification on the shaping of the surface topography, which determines the adhesion and proliferation of cells, resulting in the improvement of biocompatibility. The key issue in this content is successful implantation and long-term cardiac support. The coatings with the developed surface are supposed to initiate the formation of a scar that can limit the thromboembolic events, which is confirmed elsewhere [8]. Therefore, the premise of this surface modification is to obtain tight adhesion around the inlet cannula to the apical site of the myocardium. Secondly, it should prevent uncontrolled overgrowth into the cannula’s lumen, which could block the inflow and consequently disturb the proper operation of the device resulting finally in turbulent flow or suction events [18].
Therefore, the goal was the evaluated correlation between surface topography parameters on the susceptibility of cells to grow and adhere as a coating solution with potential for use in MCS devices. Currently, literature data do not provide any information on the impact of the proposed technology on the phenomenon of cells adhesion and proliferation. Hence the manuscript has a pioneering nature with a deep focus on the correlation of topographic parameters on biocompatibility. In medicine, progress does not occur fast, but on the basis of the evolution of the introduced solutions. Tests of surfaces used on VADs inflow cannula have been conducted for many years [39], while the milestone is the technology and proper selection of process parameters. Freels et al. [40] observed a positive effect of the textured coating on CoCr rods especially after sintering with small microspheres in the range of 100–250 µm diameter. Rods were implanted into the femoral muscle of rabbits and the formation of a permanent connection at the implant-tissue interface was observed. This research, however, was targeted at orthopaedics issues.
In this work, we proposed the use of vacuum sintering technology. We focused on the selection of the appropriate powder, considering its shape and size, ultimately obtaining a different surface topography. As highlighted in chapter 2.1 the applied detailed process parameters are covered by the secret of know-how due to the patent potential, however, they do not constitute the main considerations in the manuscript. Therefore, two powder morphologies were used, i.e. spherical and irregular. The designations S1, S2, S3 and I1, I2, I3 refer to the size of the powders used, and the roughness determined by the Ra parameter.
Based on the roughness test it was observed that an increase in Ra/Rz and Sa parameters was accompanied by an increase of the grain size of the powders used during the modification. The Ra parameter was in the range of 20–48 µm, and the Sa surface roughness parameter for the spherical modification was in the range of 28–58 µm, and for the irregular modification 25–56 µm. Thus, no significant influence of the powder morphology on the degree of surface roughness was observed. With the use of a digital microscope and scanning microscopy, a high degree of differentiation of the topography of the coatings was observed due to the morphology of the powders used, i.e. spherical and irregular powder. The coatings formed as a result of the S modification have a regular structure with rounded shapes. The modification I resulted in the formation of a coating with numerous irregular elevations and craters. Based on the cross-sectional analysis, the thickness of the formed coatings was estimated in the range of h = 265–526 µm for modification S and h = 202–735 µm for modification I. Moreover, with the use of image analysis software, it was possible to assess the degree of porosity of the obtained coatings and the dependence resulting from the influence of the powder sizes. The analysis of the coating porosity and the presence of open and closed pores as well as their size are key issues due to the potential of cells and tissue anchoring for permanent integration with the implant. The direct increase in porosity is visible along with the grain size of the powders used. In the case of the S modification, the obtained coatings are characterized by a greater porosity than the samples after modification I, in the range of 41–47%. The coatings obtained with the use of irregular powders had a porosity of 29–37%. Based on the analysis of the chemical composition of EDS, the presence of titanium (Ti Kα) was observed on the produced coating (peak with the highest intensity—4.6 keV). The tests thus confirmed the chemical composition of the applied powder—CP-Ti. Moreover, the presence of the Al and Nb alloying element peaks of the base material was observed. In order to perform a comparative analysis of the influence of modifications on the phase composition of coatings, a study with the use of X-ray diffraction was carried out. For all modifications of S and I, the highest intensity (2θ) of diffraction peaks was observed at about 47°, corresponding to the crystallographic orientation (101), of the titanium α phase. All samples after modification with the vacuum sintering method for the proposed powders are characterized by a contact angle of θ ≈ 103º ± 4, which can be attributed to the hydrophobic character. Due to the fact that the cytotoxicity assessment is one of the basic methods for determining the safety of medical devices, the direct method of cytotoxicity testing was performed in accordance with ISO 10993-5: 2009 on fibroblasts (L929 ATCC). No cytotoxic effect was observed for all modifications, and the survival rate ranged from 85 to 96% after 24 h of incubation. Since one of the first stages of the body’s reaction to the implant is the attachment of water and protein to the surface, it was important to verify the impact of the modification on protein adsorption using the example of albumin. As a result, a positive effect of the proposed surface developments on the amount of adsorbed protein in relation to the reference sample for all S and I modifications was observed. For both human whole blood and platelet-leukocyte concentrate, higher albumin concentrations were noted for the S modification using spherical powders designated S1 and S3, respectively. Samples S3 and I3 showed a similar level of albumin for whole blood of about 477 ± 10 ng/µl. The coating after the S3 modification was also characterized by the greatest susceptibility to protein binding from the concentrate, reaching a concentration of 530 ± 10 ng/µl. Thus, our protein adsorption studies have proven the dependence of surface complexity on protein adsorption. After blood incubation coatings were analysed by SEM to evaluate the method and location of cell attachment to the textured surface. As a result, the attachment of platelets and erythrocytes was observed mainly in the corners of the structures connecting adjacent powder grains. Therefore, it confirmed the necessity to determine the correlation between topography surface diversity and cells attachment in order to achieve permanent implant-tissue integration.
In the future, the presented research will be extended to the analysis of protein adsorption in a dynamic flow system, cell proliferation and in vivo research. The mechanism of pseudointimal protein layer formation is a very important phenomenon from the biocompatibility point of view, but unfortunately, it is still little understood.
5 Conclusions
-
1.
The morphological analysis led to the observation that there was no significant influence of the powders’ morphology on the degree of surface roughness. However, a high degree of diversity of the coating’s topography was observed due to the morphology of the used powders. The direct increase in porosity is visible along with the increase of powders’ grain size.
-
2.
The analysis of chemical composition in the applied powders of both morphologies confirmed the presence of Ti as the main constituent with Al and Nb alloying elements of the base material. No phase transition was detected due to the sintering process.
-
3.
When determining the intended use of the produced coatings, it should be considered that due to the high porosity and low cohesion, the coating may undergo microcracking when subjected to direct loading. Samples modified with irregular powders are characterized by a lower indentation microhardness and a lower indentation modulus of elasticity than the S-series.
-
4.
All samples after modification with the vacuum sintering method for the proposed powders showed hydrophobic character. During biological tests with cell culture, no cytotoxic effect was observed for any of the samples. Protein adsorption studies have shown high susceptibility of the developed surfaces to albumin attachment, which is crucial in the process of biofunctional layer formation.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Molina EJ, Shah P, Kierman MS, Kirklin JK. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. The Society of Thoracic Surgeons Intermacs Annual Report. 2021;111:778–92. https://doi.org/10.1016/j.athoracsur.2020.12.038.
Benjamin EJ, Blaha MJ, Chuve SE, Cushman M. Heart disease and stroke statistics – 2017 update: a report from the American Heart Association. Circulation. 2017;135:146–603. https://doi.org/10.1161/CIR.0000000000000485.
Yearly Statistics Overview Eurotransplant. 2021. https://statistics.eurotransplant.org/index.php?search_type=&search_organ=heart&search_region=by+country&search_period=2021&search_characteristic=&search_text= Accessed 30 May 2022
McMurray JJV, Adamopoulos S, Anker SD, Auricchio A. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847.
Kopernik M, Milenin A. Two-scale finite element model of multilayer blood chamber of POLVAD-EXT. Arch Civ Mech Eng. 2012;12:178–85. https://doi.org/10.1016/j.acme.2012.04.003.
Kirklin JK, Naftel DC, Pagani FD, Kormos RL. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transpl. 2015;34:1495–504. https://doi.org/10.1016/j.healun.2015.10.003.
Padera RF, Schoen FJ. Other cardiovascular devices. In: Ratner BD, Hoffman AS, editors. Biomaterials science: an introduction to materials. 3rd ed. Academic Press; 2013. p. 784–98.
Dasse KA, Chipman SD, Sherman CN, Levine AH, Frazier O. Clinical experience with textured blood contacting surfaces in ventricular assist devices. ASAIO Trans. 1987;33:418–25.
Rose EA, Levin HR, Frazier OH. Artificial circulatory support with textured interior surfaces: a counterintuitive approach to minimizing thromboembolism. Circulation. 1994;90(5 Pt 2):87–91.
Menezes P, Lovell M. Surface texture. In: Davim JP, editor. Materials and surface engineering. Woodhead Publishing Reviews: Mechanical Engineering Series; 2012. p. 207–42.
Kirklin JK, Pagani FD, Kormos RL. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–6. https://doi.org/10.1016/j.healun.2017.07.005.
Pleșoianu FA, Pleșoianu CE, Bojan IB, Bojan A, Țăruș A, Tinică G. Concept, design, and early prototyping of a low-cost, minimally invasive, fully implantable left ventricular assist device. Bioengineering. 2022;9(5):201.
Drazner MH. A new left ventricular assist device — better, but still not ideal. New Eng J Med. 2018;378(15):1442–3. https://doi.org/10.1056/NEJMe1802639.
Burrell AJC, Salamonsen R, Murphy DA. Complications of mechanical circulatory and respiratory support. In: Gregory SD, Stevens MC, Fraser JF, editors. mechanical circulatory and respiratory support. Academic Press; 2018. p. 495–528.
Castrodeza J, Ortiz-Bautista C, Fernández-Avilés F. Continuous-flow left ventricular assist device: current knowledge, complications, and future directions. Cardiol J. 2022;29(2):293–304. https://doi.org/10.5603/CJ.A2021.0172.
Breme J, Kirkpatrick CJ, Thull R. Metallic biomaterial interfaces. Wiley-VCH. 2008. https://doi.org/10.1002/9783527622603.
Najjar SS, Slaughter MS, Pagani FD, Starling RC. Analysis of pump thrombus events in patients in the HeartWare advance bridge to transplant and continued access protocol trial. J Heart Lung Transpl. 2014;33:23–34. https://doi.org/10.1016/j.healun.2013.12.001.
Kurtyka P, Kustosz R, Kaczmarek M, Tokarska K. Surface modifications for inflow cannulas of ventricular assist devices-comparison of latest solutions. Eng Biomater. 2019;151:17–23.
Glass CH, Christakis A, Fishbein GA, Watkins J. Thrombus on the inflow cannula of the HeartWare HVAD: an update. Cardiovasc Pathol. 2019;38:14–20. https://doi.org/10.1016/j.carpath.2018.09.002.
Blitz A. Pump thrombosis-A riddle wrapped in a mystery inside an enigma. Ann Cardiothorac Surg. 2014;3:450–71. https://doi.org/10.3978/J.ISSN.2225-319X.2014.09.10.
Houel R, Moczar M, Clerin V. Pseudointima in inflow conduits of left ventricular assist devices. Ann Thorac Surg. 1999;68:717–23. https://doi.org/10.1016/S0003-4975(99)00527-5.
Hecker JF, Scandrett LA. Roughness and thrombogenicity of the outer surfaces of intravascular catheters. J Biomed Mater Res. 1985;19:381–95. https://doi.org/10.1002/JBM.820190404.
Braune E, Lataur RA, Reinthaler M, Landmesser U. In vitro thrombogenicity testing of biomaterials. Adv Healthcare Mater. 2019;1900527:1–17. https://doi.org/10.1002/adhm.201900527.
Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219. https://doi.org/10.1177/0022034509359125.
Gawlikowski M, Kustosz R, Glowacki M. Non-invasive assessment of thromboembolism in rotary blood pumps: case study. In: Proceedings Volume 10455, 12th Conference on Integrated Optics: Sensors, Sensing Structures, and Methods. 2017;10455. https://doi.org/10.1117/12.2282667. Accessed 14 June 2022.
Fraser JF, Gregory SD, Stevens MC. Mechanical circulatory and respiratory support. 1st ed. London: Academic Press; 2017.
Zhang Z, Kurita H, Kobayashi H. Osteoinduction with HA/TCP ceramics of different composition and porous structure in rabbits. Oral Sci Int. 2005;2:85–95. https://doi.org/10.1016/S1348-8643(05)80011-4.
Campbell CE, von Recum AF. Microtopography and soft tissue response. J Invest Surg. 1989;2:51–74. https://doi.org/10.3109/08941938909016503.
John M, Prakash RV. Void content measurement in fiber reinforced plastic composites by X-ray computed tomography. Mat Sci Forum. 2018;928:38–44. https://doi.org/10.4028/www.scientific.net/MSF.928.38.
Kopernik M, Milenin A. Numerical modeling of substrate effect on determination of elastic and plastic properties of TiN nanocoating in nanoindentation test. Arch Civ Mech Eng. 2014;14:269–77. https://doi.org/10.1016/J.ACME.2013.10.001.
Gourlay T, Black RA. Biomaterials and devices for the circulatory system. 1st ed. Woodhead Publishing; 2010.
Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. https://doi.org/10.1006/abio.1976.9999.
Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177(2):751–66 (PMID: 18110453).
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75.
Smith PK, Krohn RI, Hermanson GT, Malia AK. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. https://doi.org/10.1016/0003-2697(85)90442-7.
Stoscheck CM. Protein assay sensitive at nanogram levels. Anal Biochem. 1987;160(2):301–5.
Frazier OH, Kar B, Patel V, Gregoric ID. First clinical use of the redesigned HeartMate II left ventricular assist system in the United States: a case report. Tex Heart Inst J. 2004;31:157–9.
Yamada Y, Nashinaka T, Mizuno T, Taenaka Y. Neointima-inducing inflow cannula with titanium mesh for left ventricular assist device. J Artif Organs. 2011;14:269–75. https://doi.org/10.1007/s10047-011-0586-4.
Yamada Y, Hoki R, Kikuchi N. Neointima-inducing inflow cannula for over 5 years after left ventricular assist device implantation. J Heart Lung Transpl. 2021;40(4):S388. https://doi.org/10.1016/j.healun.2021.01.1092.
Freels DB, Kilpatrick S, Gordon ES, Ward WG. Animal model for evaluation of soft tissue ingrowth into various types of porous coating. Clin Orthop Relat Res. 2002;397:315–22. https://doi.org/10.1097/00003086-200204000-00036.
Acknowledgements
The project is co-financed by the National Science Centre, Poland – PRELUDIUM 16 2018/31/N/ST8/01085.
The statue work Z3 was executed in the Institute of Metallurgy and Materials Science of the Polish Academy of Sciences, Cracow, Poland.
Author information
Authors and Affiliations
Contributions
RM: conceptualization; RM, PK: methodology; PK: formal analysis and investigation; RM, PK, MK, AB: writing—original draft preparation; PK, MK, AB: writing—review and editing; RM: funding acquisition; MS, PK, JW, KK, LM, RM: resources; MK, RM, MK, RK: supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kurtyka, P., Kopernik, M., Kaczmarek, M. et al. Biofunctional impact of textured coatings in the application of heart assist therapy. Archiv.Civ.Mech.Eng 23, 31 (2023). https://doi.org/10.1007/s43452-022-00573-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-022-00573-8