Abstract
Dengue fever has become one of the deadliest infectious diseases and requires the development of effective antiviral therapies. It is caused by members of the Flaviviridae family, which also cause various infections in humans, including dengue fever, tick-borne encephalitis, West Nile fever, and yellow fever. In addition, since 2019, dengue-endemic regions have been grappling with the public health and socio-economic impact of the ongoing coronavirus disease 19. Co-infections of coronavirus and dengue fever cause serious health complications for people who also have difficulty managing them. To identify the potentials of mangiferin, a molecular docking with various dengue virus proteins was performed. In addition, to understand the gene interactions between human and dengue genes, Cytoscape was used in this research. The Kyoto Encyclopedia of Genes and Genomes software was used to find the paths of Flaviviridae. The Kyoto Encyclopedia of Genes and Genomes and the Reactome Pathway Library were used to understand the biochemical processes involved. The present results show that mangiferin shows efficient docking scores and that it has good binding affinities with all docked proteins. The exact biological functions of type I interferon, such as interferon-α and interferon-β, were also shown in detail through the enrichment analysis of the signaling pathway. According to the docking results, it was concluded that mangiferin could be an effective drug against the complications of dengue virus 1, dengue virus 3, and non-structural protein 5. In addition, computational biological studies lead to the discovery of a new antiviral bioactive molecule and also to a deeper understanding of viral replication in the human body. Ultimately, the current research will be an important resource for those looking to use mangiferin as an anti-dengue drug.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dengue fever is a serious viral disease that is ubiquitous in tropical and subtropical regions (Guzman and Harris 2015). According to the World Health Organization, 100 million cases of dengue fever occur every year. In 500,000 of these cases, hospitalization is required and in 25,000 cases, conditions worsen and lead to death. According to a recent report, there are 390 million dengue infections worldwide each year, more than three times the estimates of the World Health Organization (Qamar et al. 2019). This mosquito-borne virus disease is endemic in over 100 countries and triggered by the dengue virus transmitted through two vectors such as Aedes aegypti and Aedes albopictus (Vega-Rua et al. 2014).
This virus infection is known by common clinical symptoms such as acute high fever, headache, severe myalgia, ostalgia and arthralgia, rash, risk of bleeding, and low white blood cell counts after infection (Gubler and Clark 1995). Similarly, dengue hemorrhagic fever (DHF) is a dengue infection condition that causes extremely high fever, heavy bleeding, shock, low platelet count, pachyhematosis, and a high mortality rate (Gubler 1998; Gao et al. 2018). Similar to the current pandemic, it was once considered the most popular, contagious, and deadliest arboviral disease in the world. There has been no drug or vaccine against the dengue virus for several years (Schul et al. 2007; Latif et al. 2020). Therefore, there is an urgent need to find herbal medicines for dengue fever that are free from side effects.
Many pharmacologically active metabolites have been isolated from medicinal plants and microbes for decades. These natural metabolites cause minimal or no side effects when used for short- or long-term use (Hu et al. 2013). In addition, the components of some medicinal plants have antiviral properties (Calland et al. 2012). Particularly, mangiferin is a bioactive molecule found in higher plants that is attracting the attention of the pharmaceutical industry due to its pharmacological potential (Imran et al. 2017). It is classified as xanthone glucoside and is considered to be the predominant metabolite of members of the genus Mangifera.

Mangiferin (1) is widespread in many different parts of Mangifera indica L., Anacardiaceae, including the leaves, bark, and fruit parts such as peels, stems, stalks, and stones (Imran et al. 2017). Previous studies have shown that mangiferin has potential pharmacological properties such as analgesic, anti-aging, anti-cancer, anti-diabetic, anti-oxidant, anti-proliferative, anti-viral, cardiotonic, diuretic, hepatoprotective, and immunomodulatory effects (Khare, 2016; Du et al. 2018). However, such extensive studies are not available in countries with a strong background in the use of medicinal plants to treat certain viral diseases such as dengue, SARS-CoV-2, and poliovirus. Although synthetic drugs have reduced the use of herbal treatments, this research underscores the essential role of computational biology in testing such plant molecules in order to explore their therapeutic potential in severe acute viral diseases.
A recent case report states that the tropical and subtropical diseases such as dengue, zika, chikungunya viruses, and scrub typhus should also be considered deadly diseases during a corona pandemic, since co-infection of two different viruses changes the dynamics and natural history of the disease in the human body which also leads to mortality; hence, it is necessary to focus on that (Hilmy et al. 2019). Particularly, doctors should exercise extreme caution when giving fluids intravenously to COVID-19 patients co-infected with dengue, as it has been reported to cause pulmonary edema in COVID-19 patients. They reported that it may be due to the possibility of cross-immunity between these two viruses. Therefore, in the present study, the support of computer biology was sought to find new drug candidates for such viral diseases’ complications.
Genomes of Dengue Virus
Dengue serotypes are divided into five groups: DENV-1, DENV-2, DENV-3, DENV-4, and DENV-5. The first four serotypes are somewhat similar to the 65% of the sequence, although they bind uniquely to antibodies in human blood serum (Mustafa et al. 2015). Despite their genetic differences, these four serotypes cause the same diseases in their hosts (Qamar et al. 2019). According to the report of Mustafa et al. (2015), alike other dengue serotypes, the exact reason for the occurrence and transmission of DENV-5 is not clear. However, unlike the other four dengue serotypes that are transmitted between people, it primarily circulates among non-human primates and follows the sylvatic cycle (Mustafa et al. 2015).
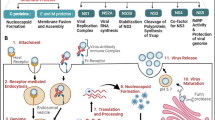
In general, the polypeptides of dengue virus are encoded by a longer single-stranded RNA which is called positive-sense RNA and is classified into structural proteins including membrane (M), envelope (E), and capsid (C) and non-structural proteins including non-structural protein 1 (NS1), non-structural protein 2A (NS2A), non-structural protein 2B (NS2B), non-structural protein 3 (NS3), non-structural protein 4A (NS4A), non-structural protein 4B (NS4B), and non-structural protein 5 (NS5) (Endy et al. 2011; Normile 2013; Qaddir et al. 2017). Figure 1 precisely shows the classified dengue proteins.
Dengue virus: Transmission and its genome structural organization. It reveals three structural proteins, namely C (capsid), PrM (premembrane), and E (envelope) and 7 non-structural proteins, namely NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. This was created in BioRender.com
Materials and Methods
Docking and Modeling Platform
This in silico approach was carried out with Maestro V.12.7 modules such as LigPrep, grid generation, SiteMap, and Glide XP docking. This valuable software was installed in Centos Linux, which served as the operating system for this docking research. Almost all the methods of computational drug design followed our previous publications (Kalaimathi et al. 2021; Rani et al. 2022; Prabhu et al. 2022).
Biological Data
In this computational biological research, mangiferin (1) was used as a ligand against three different types of dengue virus enzymes to assess its effectiveness. This was found in a chemical database and collected from it as a mol file (www.chemsipder.com). The selected dengue enzymes such as the RNA-dependent RNA polymerase (RdRp) domain from NS5 (2J7U), envelope glycoprotein from DENV3 (1UZG), and the NS2B/NS3 protease from DENV1 (3L6P) were included in the protein database and their alphanumeric identities taken from the database are 2J7U, 1UZG, and 3L6P (www.rcsb.com).
Targets Preparation
All dengue enzymes were subjected to enhanced experiments using the Maestro V 12.7 protein preparation wizard tool. It was carried out to address the issue of missing side and back chains in those enzymes. This was applied in accordance with Prabhu et al. (2017). In making this process, we face two main courses: preparation and refinement. Typically, the protein molecules (X-ray crystallographic structure) are bound and tangled with water molecules; this state is unsuitable for docking. As a result, it was removed from those enzymes during the protein preparation process. This protein preparation process was completed by the optimization and minimization gears and it finally gave us the final shape of the prepared protein to find the grid site (drug binding site). This technique was applied in accordance with Prabhu et al. (2018).
Active Site Prediction
This site validation plays a crucial role in the docking study. After the site has been thoroughly examined, it shows the drug binding pouch, the areal volume along it, and the rating of the site. An active site for grid generation was predicted with the sitemap generation tool in Maestro V12.7. Finally, only one location for grid generation was selected based on its location scores and volume. This technique was carried out according to Vijayakumar et al. (2018).
Grid Generation
It was used to stabilize the binding site of dengue enzymes. In grid-based ligand docking, a small molecule is docked to the specific protein in order to analyze the docking parameters. With this approach, a grid box was created to dock the small molecule at the focal point of dengue proteins. The grid box of 1UZG was built with X: –3.55, Y: 10.94, and Z: 67.39. Likewise, the grid box of 2J7U was built with X: 27.75, Y: 75.54, whereas the grid box of 3L6P was built with X: –31.8, Y: –11.03, and Z: 28.24. Finally, the macromolecules are ready to dock with the selected small molecule. This technique was applied in accordance with Prabhu et al. (2017).
Ligand Preparation
The small molecule was also made using the LigPrep (2.4) module prior to the docking action. Here the force field Optimized Potentials for Liquid Simulations 2005 (OPLS2005) was used to optimize the geometry of the drawn ligand. This module was used to build up the ligand as 3D structures (Fig. 2) from 1D (Smiles) and 2D (SDF) representations by combining the tools; tautomers and stereoisomers are also examined in order to reduce the geometric complexity of the ligand. Ultimately, a ligand molecule made with a molecular weight or a certain number and type of functional groups with correct chiralities for each successfully processed input structure was ready to be docked to the particular proteins. This technique was applied in accordance with Vijayakumar et al. (2018).
Molecular Docking
First, a ligand molecule was docked to the dengue proteins with the Grid Glide docking module from Maestro V.12.7 in order to test the docking parameters. We predicted the binding affinities and inhibitory constants of the ligand to the proteins using Standard Precision (SP) and Xtra Precision (XP) docking modes. Both docking modes were used to assess the flexibility of the ligand with the target. In this case, the drug-target molecule shows potential contributions such as docking scores, hydrophobic interactions, hydrogen bond interactions (side and back chain), pi-pi stacking, and salt bridge contacts. This was applied in accordance with Vijayakumar et al. (2016a, b).
Network Construction and Pathway Enrichment
The network was built using Cytoscape v3.8.0. This technique has been used to evaluate and manipulate dengue serotype interactions between viral components and viral genes. Dengue datasets were retrieved from the DenHunt website to find the relationships between viral components and human genes. The KEGG database was also used to identify dengue virus signaling pathways and we looked for genes that lead to the activation of antiviral enzymes. We used the website https://reactome.org/ to understand the intrinsic mechanisms of the immune system involved in antiviral enzyme production to find out exactly what happens when interferon genes including IFN-α and IFN-β are stimulated. These enrichment analysis results were screened at p < 0.05.
Mangiferin Drug Probability
The pass server was used to assess the drug potential of mangiferin (1) in accordance with our previous articles, where it was subjected to know its range of probability of being active and probability of being inactive in the enzymes in relation to drug potential (Kalaimathi et al. 2021).
Pharmacokinetics analysis
A server pkCSM was used to assess the various physicochemical and pharmacokinetic properties of mangiferin (Rani et al. 2022).
Results and Discussion
The ultimate goal of all dengue virus research is to identify the pathogenesis of dengue infection and establish successful therapeutic interventions to suppress viral infection and replication in order to avoid disease progression to extreme types such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). Dengue-host interaction data, which provides current information on dengue disease at the molecular level, would be useful for future research on dengue (Silvestre et al. 2021). As noted in previous research, this research will enable us to understand how dengue virus replicates in the cell and how host-virus interactions suppress dengue replication and resolve disputes over viral pathogenesis (Karyala et al. 2016).
Computer-Aided Drug Design
Ligand-Binding Cavities of Dengue Proteins
The site prediction approach had disclosed up to four prominent binding pockets on protein molecules. Here, the site analysis revealed the residues consistency of three dengue proteins such as 1UZG, 2J7U, and 3L6P. Firstly, the ligand binding cavity of RNA-dependent RNA polymerase (RdRp) of NS5 dengue protein possesses forty residues including 279, 450, 345, 302, 346, 347, 348, 350, 351, 354, 355, 358, 359, 571, 575, 576, 577, 579, 536, 299, 537, 600, 538, 601, 539, 602, 580, 581, 582, 477, 586, 587, 588, 480, 481, 592, 597, 449, 598, and 599 (Table S1). Secondly, the ligand binding cavity of dengue virus type 3 envelope glycoprotein molecule possesses thirty residues including 147, 148, 351, 352, 353, 354, 173, 174, 355, 150, 175, 356, 176, 357, 358, 156, 157, 158, 361, 362, 39, 363, 40, 142, 143, 144, 292, 145, 146, and 293 (Table S2). Finally, the ligand binding cavity of dengue virus 1 NS2B/NS3 protease molecule possesses twenty-four residues including 123, 124, 202, 203, 204, 170, 171, 172, 197, 173, 174, 175, 212, 213, 214, 137, 215, 138, 216, 139, 217, 166, 168, and 169 (Table S3). Since all interactions with residues originate from this binding cavity, it plays an important role in molecular docking research. It also shows the electrostatic interactions between the small molecule and the protein. Apart from that, in most significant cases, it can help to determine the higher affinity of the ligand and the exact distance from the target position (Prabhu et al. 2017).
Molecular Docking Simulations
The efficiency of mangiferin (1) with various dengue proteins using molecular docking was evaluated. These docking studies provided docking metrics such as docking score, electrostatic energy, and hydrogen bond interactions. The functional groups of mangiferin showed positive binding affinities with decent H-bond distance values with dengue protein residues. In addition, the binding affinities indicate how much the ligand is contributing to the target and how flexible it is. This study found that mangiferin is an active biomolecule for dengue complications. We came to this conclusion after going through the docking parameters in detail as follows.
Mangiferin with RNA-dependent and RNA polymerase (RdRp) of NS5 dengue protein
With this docking, mangiferin has pretty good docking values of –9.868 (Table 1). It has the potential to be used as an active antiviral drug for the envelope glycoprotein DENV 3. The docked complex was studied to understand the interactions between ligands and dengue protein residues. This study was shown to have ligand binding relationships with dengue protein residues such as LYS578, THR346, and GLY601 (Fig. 2a). It was known that LYS578 and THR346 have covalent binding interactions with mangiferin. LYS578 shows covalent H-bond contacts with the distances of 2.56 and 1.90 Å. Likewise, THR346 shows covalent H-bond contacts with the distances of 1.99 and 2.42 Å. GLY601 was found to have an H-bond contact with a distance of 2.11 Å (Fig. 2a and Table 2). The amino acid contacts with the functional groups of mangiferin are shown in the two-dimensional diagrams. Almost all residues have hydrogen bonds to the OH groups of the ligand (Fig. 2b).
Mangiferin with DENV 3 envelope glycoprotein
Mangiferin has a good docking score of –9.729 (Table 1). It has the potential to be used as an active antiviral drug for the envelope glycoprotein DENV 3. It shows hydrogen bond contacts with this protein residue such as PRO354, ASN353, LEU349, ALA35, and PHE335 (Fig. 3a). In particular, ASN353 was covalently bound with the H-bond distances of 2.70 and 2.43 Å. The hydrogen bond distances of the remaining residues such as PRO354, LEU349, ALA35, and PHE335 were found to be 1.72, 2.64, 2.64, and 2.29 Å (Fig. 3a and Table 2). The amino acid contacts with the functional and oxygen groups of mangiferin are shown in the two-dimensional diagrams. The residues PRO354, ASN353, ALA35, and PHE335 showed hydrogen bonds with the OH groups of the ligand, while the backbone chains of ASN353 and LEU349 showed hydrogen bonds with the O groups of the ligand (Fig. 3b). ASN 353 has two compounds as a functional group and one of them is an oxygen group (Fig. 3b).
Mangiferin with DENV1-NS2B/NS3 protease
Mangiferin has a docking score of –9.876, indicating that it is a promising drug candidate for this dengue protein–related disease complication. Additionally, it has the potential to be used as an active antiviral drug for the DENV1-NS2B/NS3 protease. It has higher level docking metrics like docking score and floating energy values as shown in Table 1. The docked complex and the interactions of the residues with the ligand atoms were examined. It shows H-bond contacts with this protein residue such as GLU170, SER138, TRP139, and ILE215 (Fig. 4a). The hydrogen bond distances of these residues were found to be 1.91, 1.95, 2.35, and 1.73 Å (Fig. 4a and Table 2). Full interactions were framed by backbone hydrogen-bond contacts. The results of the two-dimensional interaction show that almost all residues have hydrogen bonds with the OH groups of the ligand (Fig. 4b).
Mangiferin was the first xanthone to be therapeutically beneficial in the treatment of herpes virus infections. It performs a variety of biochemical functions that are useful for the body (Negi et al. 2013). Generally, the xanthones inhibit the HIV-1 virus transcriptase enzyme, which is reported to be a key function of xanthine (Freddy and Ericsson 2015). Computer research in 2015 showed that xanthones consist of the right essential metabolites to obtain antiviral drugs (Umar et al. 2021). Recently, a study by Umar et al. (2021) reported that mangiferin has high plasma protein binding potential and is not a substrate for p-glycoprotein. Finally, they pointed out that mangiferin can be delivered to its destination in a reasonable quantity due to its water solubility.
Mangiferin Drug Probabilities
This prediction revealed that mangiferin has a broad spectrum of drug probabilities, finding that it has the drug probabilities to be active in more than 30 enzymatic mechanisms, with the p-value > 0.702. In Table 3, it was clearly summarized.
Drug-Likeness and Pharmacokinetics Parameters
Mangiferin physicochemical parameters were found to have a molecular weight of 422.342, LogP value of -0.7165 with 2 rotatable bonds, 8 acceptors, and 11 donors, and a surface area of 166.412. Similarly, the prediction of the pharmacophoric analysis indicated that mangiferin exhibits a broad spectrum of pharmacokinetic properties as shown in Table 4.
Network Construction and Analysis
The majority of the direct contacts recorded in the literature were calculated using affinity purification, pull-down assays, microscopic co-localization, or high-throughput approaches such as yeast two-hybrid (Y2H), and tandem affinity purification, accompanied by mass spectrometry. Dengue virus protein PPIs in mosquitoes and human hosts have been estimated in some studies using computer modeling that focused on structural similarities of proteins. Protein-predicted interactions from such publications were only collected when the human protein was previously shown to be significant in dengue virus infection. Figure S1 shows that the dengue genomes comprised 26 nodes with 703 edges in the network. The dengue genomes such as DENV1, DENV2, DENV3, DENV4, DHF, DENV-RNA, M, E, C, prM, 5′-UTR, 3′-UTR, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 are considered to be the primary serotypes of dengue infection. These 26 proteins are promising targets for the treatment of dengue infection and should be further investigated. Figure S2 shows 452 nodes with 727 edges. Eleven dengue serotypes are involved in the association of human genes as a whole. Among the viral components, 5′- and 3′-UTR, DENV-RNA consists of 31 interactions, capsid protein (C) consists of 66 interactions, envelope (E) protein consists of 34 interactions, premembrane (prM) protein consists of 24 interactions, non-structural protein-1 (NS1) consists of 162 interactions, non-structural protein 2A (NS2A) consists of 43 interactions, non-structural protein 2B (NS2B) consists of 43 interactions, non-structural protein 3 (NS3) consists of 23 interactions, non-structural protein 4A (NS4A) consists of 126 interactions, and non-structural protein 4B (NS4B) also consists of 15 interactions (Table 5).
Pathway Enrichment Analysis
The KEGG software was used to know the enzymatic pathways of dengue virus. Since certain families of pattern recognition receptors are responsible for detecting viral pathogens and triggering innate immune responses, we investigated signaling pathway enrichment in this area. Foreign RNA that enters a cell as a result of intracellular virus replication is recognized by RIG-I-like receptors, a family of cytosolic RNA helicases (RLRs). RLR proteins such as RIG-I, MDA5, and LGP2 are present in both immune and non-immune cells. RLRs use various intracellular adapter proteins to activate signaling pathways that contribute to the synthesis of type I interferon and other inflammatory cytokines that are essential for virus exclusion (Fig. S3). We used the Reactome website to discover the enzymatic role of type I interferon in the immune system, which was discovered as a result of the KEGG study results. The exact biological pathways of type I interferon are shown in Fig. 5a and b. In general, it is believed that the interferons (IFNs) are signaling molecules that are important in activating immune responses, particularly the antiviral and antitumor potential. Interferons are classified into three types, i.e., type I, type II, and type III. This module mainly focuses on type I IFNs alpha and beta.
Conclusions
Antiviral drugs are urgently needed around the world to treat arboviral infections and co-infections with other viral infections. Therefore, the technological interventions are required to dissect the constituents from natural sources such as medicinal plants, microbes, and algae and identify suitable molecules that could serve as potential drugs to treat viruses. Particularly, dengue and coronavirus are two well-known infectious diseases with high levels of contagious outbreaks. Silvestre et al. (2021) reported that dengue virus improves human immunity to COVID-19 in people who are already infected with dengue virus. Previous in vitro and in silico studies showed that mangiferin has broad antiviral potential for viruses such as herpes simplex virus-1, human immune virus-1, and SARS-CoV-2. In this computer-assisted biological study, it was demonstrated that mangiferin is also an active ingredient potential for all docked dengue proteins. Based on previous virus studies and the current report, mangiferin would undoubtedly serve as an important antiviral drug for viral complications. However, this research suggests that much more pharmacological studies are also needed to evaluate their toxicity and other therapeutic options. In addition, this pharmacological network analysis will enable the development and computation of a crucial map of the hierarchical interaction networks that exist in humans during the viral life cycle. This manuscript, particularly the section on network pharmacology, will add to a deeper understanding of the dispute between dengue and its human host. Ultimately, we hope that this finding will help in the development of an antiviral drug in this pandemic situation.
References
Calland N, Dubuisson J, Rouillé Y, Séron K (2012) Hepatitis C virus and natural compounds: a new antiviral approach? Viruses 4:2197–2217. https://doi.org/10.3390/v4102197
Du S, Liu H, Lei T, Xie X, Wang H, He X, Tong R, Wang Y (2018) Mangiferin: an effective therapeutic agent against several disorders. Mol Med Rep 18:4775–4786. https://doi.org/10.3892/mmr.2018.9529
Endy TP, Anderson KB, Nisalak A, Yoon IK, Green S, Rothman AL, Thomas SJ, Jarman RG, Libraty DH, Gibbons RV (2011) Determinants of inapparent and symptomatic dengue infection in a prospective study of primary school children in Kamphaeng Phet, Thailand. PLoS Negl Trop Dis 5:e975. https://doi.org/10.1371/journal.pntd.0000975
Freddy AB, Ericsson CB (2015) Molecular docking and multivariate analysis of xanthones as antimicrobial and antiviral agents. Molecules 20:13165–13204. https://doi.org/10.3390/molecules200713165
Gao B, Zhang J, Xie L (2018) Structure analysis of effective chemical compounds against dengue viruses isolated from Isatis tinctoria. Can J Infect Dis Med Microbiol 2028:3217473. https://doi.org/10.1155/2018/3217473
Gubler DJ (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11:480–496. https://doi.org/10.1128/CMR.11.3.480
Gubler DJ, Clark GG (1995) Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerg Infec Dise 1:55–57. https://doi.org/10.3201/eid0102.952004
Guzman MG, Harris E (2015) Dengue. Lancet 385:453–465. https://doi.org/10.1016/S0140-6736(14)60572-9
Hu Q-F, Zhou B, Huang JM, Gao XM, Shu LD, Yang GY, Che CT (2013) Antiviral phenolic compounds from Arundina gramnifolia. J Nat Prod 76:292–296. https://doi.org/10.1021/np300727f
Kalaimathi K, Thiyagarajan G, Vijayakumar S, Bhavani K, Karthikeyan K, Rani JM, Dass K, Sureshkumar J, Prabhu S (2021) Molecular docking and network pharmacology-based approaches to explore the potential of terpenoids for Mycobacterium tuberculosis. Pharmacol Res Modern Chinese Med 1:100002. https://doi.org/10.1016/j.prmcm.2021.100002
Karyala P, Metri R, Bathula C, Yelamanchi SK, Sahoo L, Arjunan S, Sastri NP, Chandra N (2016) DenHunt, a comprehensive data base of the intricate network of dengue-human interactions. PLoS Negl Trop Dis 10:e0004965. https://doi.org/10.1371/journal.pntd.0004965
Khare P, Shanker K (2016) Mangiferin: A review of sources and interventions for biological activities. BioFactors 42:504–514. https://doi.org/10.1002/biof.1308
Latif A, Ashiq K, Ashiq S, Ali E, Anwer I, Qamar S (2020) Phytochemical analysis and in vitro investigation of anti-inflammatory and xanthine oxidase inhibition potential of root extracts of Bryophyllum pinnatum. J Anim Plant Sci 30:219–228. https://doi.org/10.36899/japs.2020.1.0025
Mustafa MS, Rasotgi V, Jain S, Gupta V (2015) Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med J Armed Forces India 71:67–70. https://doi.org/10.1016/j.mjafi.2014.09.011
Negi JS, Bisht VK, Singh P, Rawat MSM, Joshi GP (2013) Naturally occurring xanthones: chemistry and biology. J Appl Chem 2013:621459–621459. https://doi.org/10.1155/2013/621459
Normile D (2013) Surprising new dengue virus throws a spanner in disease control efforts. Science 342:415. https://doi.org/10.1126/science.342.6157.415
Prabhu S, Vijayakumar S, Manogar P, Maniam GP, Govindan N (2017) Homology modeling and molecular docking studies on type II diabetes complications reduced PPARγ receptor with various ligand molecules. Biomed Pharmacother 92:528–535. https://doi.org/10.1016/j.biopha.2017.05.077
Prabhu S, Vijayakumar S, Praseetha P (2022) Cyanobacterial metabolites as novel drug candidates in corona viral therapies: a review. Chronic Dis Transl Med 2022:1–12. https://doi.org/10.1002/cdt3.11
Prabhu S, Vijayakumar S, Swaminathan K, Manogar P (2018) Anti-diabetic activity of quercetin extracted from Phyllanthus emblica L. fruit: in silico and in vivo approaches. J Pharm Anal 8:109–118. https://doi.org/10.1016/j.jpha.2017.10.005
Qaddir I, Rasool N, Hussain W, Mahmood S (2017) Computer-aided analysis of phytochemicals as potential dengue virus inhibitors based on molecular docking, ADMET and DFT studies. J Vector Borne Dis 54:255–262. https://doi.org/10.4103/0972-9062.217617
Qamar MT, Maryam A, Muneer I, Xing F, Ashfaq U, Ahmed Khan F, Anwar F, Geesi MH, Khalid RR, Abdul Rauf S, Siddiqi AR (2019) Computational screening of medicinal plant phytochemicals to discover potent pan-serotype inhibitors against dengue virus. Sci Rep 9:1433. https://doi.org/10.1038/s41598-018-38450-1
Rani JM, Kalaimathi K, Vijayakumar S, Varatharaju G, Karthikeyan K, Thiyagarajan G, Bhavani K, Manogar P, Prabhu S (2022) Anti-viral effectuality of plant polyphenols against mutated dengue protein NS2B47-NS3: a computational exploration. Gene Repor 27:101546. https://doi.org/10.1016/j.genrep.2022.101546
Schul W, Liu W, Xu HY, Flamand M, Vasudevan SG (2007) A dengue fever viremia model in mice shows reduction in viral replication and suppression of the inflammatory response after treatment with antiviral drugs. J Infect Dis 195:665–674. https://doi.org/10.1086/511310
Silvestre OM, Costa LR, Lopes BVR, Barbosa MR, Botelho KKP, Albuquerque KLC, Souza AGS, Coelho LA, de Oliveira AJ, Barantini CB, Neves SAVM, Nadruz W, Maguire JH, Fernandes-Silva MM (2021) Previous dengue infection and mortality in coronavirus disease 2019 (COVID-19). Clin Infec Dis 73:e1219–e1221. https://doi.org/10.1093/cid/ciaa1895
Umar HI, Josiah SS, Saliu TP, Jimoh TO, Ajayi A, Danjuma JB (2021) In-silico analysis of the inhibition of the SARS-CoV-2 main protease by some active compounds from selected African plants. J Taib Uni Med Sci 16:162–176. https://doi.org/10.1016/j.jtumed.2020.12.005
Vega-Rua A, Zouache K, Girod R, Failloux AB, Lourenco-de-Oliveira R (2014) High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J Virol 88:6294–6306. https://doi.org/10.1128/JVI.00370-14
Vijayakumar S, Manogar P, Prabhu S (2016a) Potential therapeutic targets and the role of technology in developing novel cannabinoid drugs from cyanobacteria. Biomed Pharmacother 83:362–371. https://doi.org/10.1016/j.biopha.2016.06.052
Vijayakumar S, Prabhu S, Rajalakhsmi S, Manogar P (2016b) Review on potential phytocompounds in drug development for Parkinson disease: a pharmacoinformatic approach. Inform Med Unlock 5:15–25. https://doi.org/10.1016/j.imu.2016.09.002
Vijayakumar S, Sathiya M, Arulmozhi P, Prabhu S, Manogar P, Vinothkannan R, Parameswari N (2018) Molecular docking and ADME properties of bioactive molecules against human acid-beta-glucosidase enzyme, cause of Gaucher’s disease. In Silico Pharmacol 6:3. https://doi.org/10.1007/s40203-018-0039-3
Acknowledgements
Each author expresses his gratitude to their institutions for providing the opportunity to develop this manuscript. Sincere thanks go to the co-authors for their contribution. The authors sincerely thank the anonymous reviewers for the improvement in the quality of the manuscript.
Author information
Authors and Affiliations
Contributions
KK and MJR assisted in molecular docking and network pharmacology studies. SV and KK assisted in drawing the outline for the research and collected papers on dengue and helped identify the pathway analysis enrichment. NP took formal analysis. GT and KB took part in language revision and polishing of the manuscript. SP took part in writing and structuring the manuscript, also functioning as the mentor to this research. GV helped collect the ligand drug potential. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Supplementary Information
ESM 1
(PDF 593 kb)
Rights and permissions
About this article
Cite this article
Kalaimathi, K., Rani, J.M.J., Vijayakumar, S. et al. Anti-dengue Potential of Mangiferin: Intricate Network of Dengue to Human Genes. Rev. Bras. Farmacogn. 32, 410–420 (2022). https://doi.org/10.1007/s43450-022-00258-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-022-00258-6