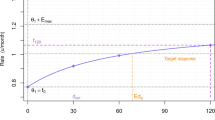
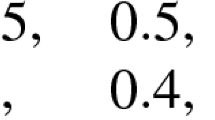Abstract
The dose-response curve has been studied extensively for decades. However, most of these methods ignore intermediate measurements of the response variable and only use the measurement at the endpoint. In early phase trials, it is crucial to utilize all available data due to the smaller sample size. Simulation studies have shown that the longitudinal dose-response surface model provides a more precise parameter estimation compared to the traditional dose response using only data from the primary time point. However, the current longitudinal models with parametric assumptions assume the treatment effect increases monotonically over time, which may be controversial to reality. We propose a parametric non-monotone exponential time (NEXT) model, an enhanced longitudinal dose-response model with greater flexibility, capable of accommodating non-monotonic treatment effects and making predictions for longer-term efficacy. In addition, the estimator for the time to maximum treatment effect and its asymptotic distribution have been derived from NEXT. Extensive simulation studies using known data-generating models and using real clinical data showed the NEXT model outperformed the existing monotone longitudinal models.




Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R &D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9(3):203–14.
DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R &D costs. J Health Econ. 2016;47:20–33.
Ting N. Dose Finding in Drug Development. New York: Springer; 2006.
Fu H, Manner D. Bayesian adaptive dose-finding studies with delayed responses. J Biopharm Stat. 2010;20(5):1055–70.
Li W, Fu H. Bayesian optimal adaptive designs for delayed-response dose-finding studies. J Biopharm Stat. 2011;21(5):888–901.
Qu Y, Liu Z, Fu H, Sethuraman S, Kulkarni PM. Modeling the impact of preplanned dose titration on delayed response. J Biopharm Stat. 2019;29(2):287–305.
Qu Y. Can a multiple ascending dose study serve as an informative proof-of-concept study? Stat Med. 2019;38(3):354–62.
Marso SP, Hardy E, Han J, Wang H, Chilton RJ. Changes in heart rate associated with exenatide once weekly: pooled analysis of clinical data in patients with type 2 diabetes. Diabetes Ther. 2018;9(2):551–64.
Tan MH, Baksi A, Krahulec B, Kubalski P, Stankiewicz A, Urquhart R, et al. Comparison of pioglitazone and gliclazide in sustaining glycemic control over 2 years in patients with type 2 diabetes. Diabetes Care. 2005;28(3):544–50.
Wilding JP, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002.
Funding
We thank Shanthi Sethuraman who provided insightful comments that greatly improved the manuscript. We also want to thank the editorial review provided by Dana Schamberger and Antonia Baldo.
Author information
Authors and Affiliations
Contributions
RD, KC, YD, PMK, and YQ made substantial contribution to the conception, design of the work. Ran designed computer programs and implement the analysis. KC and YD validated the results and they also prepared and created the interpretation of the analysis results and drafted the work and revised the paper critically for important intellectual content. All authors were dedicated to questions related to the accuracy and integrity of any part of the work were appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
This research was done when all authors were employees of Eli Lilly and Company. The authors have nothing to disclose as there is no conflict of interest for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Proof of the Asymptotic Distribution of the Estimator for \(t_p\)
The time to maximum treatment effect, \(t_p\), can be calculated by solving the following equation
but it cannot be expressed analytically in a closed form. Therefore, the derivation of the asymptotic distribution of the estimator for \(t_p\) is not trivial. Assuming the parameter \({{\varvec{\beta }}}= (\beta _1, \beta _2)'\) can be estimated consistently with
the estimator for \(t_p\) can be obtained by solving the equation
where
The asymptotic distribution of \({\hat{t}}_p\) can be derived by Taylor expansion on g with respect to t.
Since \(g({\hat{t}}_p,\hat{\beta })\) = 0, we have
where
By delta method, we have
where \(\Sigma _{\beta }\) is the variance matrix of \(\hat{\beta }\) and
Given \(g'_{t}(t_p,\beta )\) is a continuous function, by the Continuous Mapping Theorem, we have
Then, by Slutsky’s Theorem, the asymptotic distribution of \(\hat{t_p}\) can be written as
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duan, R., Chen, K., Du, Y. et al. Non-monotone Exponential Time (NEXT) Model for the Longitudinal Trend of a Continuous Outcome in Clinical Trials. Ther Innov Regul Sci 58, 127–135 (2024). https://doi.org/10.1007/s43441-023-00576-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-023-00576-4