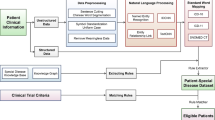
Abstract
Background:
Delays in clinical trial enrollment and difficulties enrolling representative samples continue to vex sponsors, sites, and patient populations. Here we investigated use of an artificial intelligence-powered technology, Mendel.ai, as a means of overcoming bottlenecks and potential biases associated with standard patient prescreening processes in an oncology setting.
Methods:
Mendel.ai was applied retroactively to 2 completed oncology studies (1 breast, 1 lung), and 1 study that failed to enroll (lung), at the Comprehensive Blood and Cancer Center, allowing direct comparison between results achieved using standard prescreening practices and results achieved with Mendel.ai. Outcome variables included the number of patients identified as potentially eligible and the elapsed time between eligibility and identification.
Results:
For each trial that enrolled, use of Mendel.ai resulted in a 24% to 50% increase over standard practices in the number of patients correctly identified as potentially eligible. No patients correctly identified by standard practices were missed by Mendel.ai. For the nonenrolling trial, both approaches failed to identify suitable patients. An average of 19 days for breast and 263 days for lung cancer patients elapsed between actual patient eligibility (based on clinical chart information) and identification when the standard prescreening practice was used. In contrast, ascertainment of potential eligibility using Mendel.ai took minutes.
Conclusions:
This study suggests that augmentation of human resources with artificial intelligence could yield sizable improvements over standard practices in several aspects of the patient prescreening process, as well as in approaches to feasibility, site selection, and trial selection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Acknowledgments
We would like to acknowledge Wesley Cheung and Tony LaMacchia, CBCC, for their contributions to this research.
Funding
Mendel research funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Dr Calaprice-Whitty reports personal fees from the company whose system is being evaluated in this paper. Dr Galil owns stock options and is an employee in the company whose system is being evaluated in this paper. Mr Jimenez owns stock options and is an employee in the company whose system is being evaluated in this paper. Dr Salloum owns stock options and is an employee in the company whose system is being evaluated in this paper. Mr Zariv owns stock options and is an employee in the company whose system is being evaluated in this paper.
Rights and permissions
About this article
Cite this article
Calaprice-Whitty, D., Galil, K., Salloum, W. et al. Improving Clinical Trial Participant Prescreening With Artificial Intelligence (AI): A Comparison of the Results of AI-Assisted vs Standard Methods in 3 Oncology Trials. Ther Innov Regul Sci 54, 69–74 (2020). https://doi.org/10.1007/s43441-019-00030-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-019-00030-4