Abstract
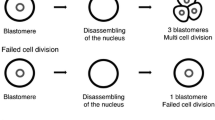
This study examined blastomere exclusion which is seen during embryo development and could represent imperfect cell division or a mechanism of aneuploidy correction. This was a retrospective cohort study which included embryos cultured in a time-lapse incubator undergoing preimplantation genetic testing for aneuploidy (PGT-A) with trophectoderm biopsy. Embryos were evaluated for blastomere exclusion early in development, late in development, both, or neither. Blastomere exclusion was compared to embryo ploidy. Embryos with no blastomere exclusion had an aneuploidy rate of 52.9%, while embryos displaying blastomere exclusion at any stage had an aneuploidy rate of 68.5% (p < .001). Early blastomere exclusion was not significantly associated with an increased aneuploidy risk (59.2% vs. 52.9% in no blastomere exclusions; p = 0.22). However, embryos with late blastomere exclusion were significantly more likely to be aneuploid, compared to embryos with no blastomere exclusions (77.5% vs. 52.9%; p < 0.001) as were embryos with both early + late blastomere exclusions (71.2% vs. 52.9%; p < 0.001). Upon restricting the analysis to aneuploid embryos, the presence of any blastomere exclusion was not significantly associated with complex aneuploidy, defined as 2 more affected chromosomes (43.9% vs. 38.7%; p = 0.28). However, the proportion with adverse embryo genetics significantly increased with the timing of blastomere exclusion (38.7%, 37%, 45.5%, and 50% for none, early, late, and early + late; p = 0.043). Late blastomere exclusion or a combination of both early + late blastomere exclusion was associated with an increased risk of aneuploid embryo genetics. Embryo selection using time-lapse culture systems should incorporate these findings when untested embryos are transferred.

Similar content being viewed by others
Data Availability
Internal Database.
Code Availability
Not applicable.
References
Technology PCoSfAR, Medicine PCoASfR. Elective single-embryo transfer. Fertil Steril. 2012;97:835–42.
Cornelisse S, Zagers M, Kostova E, Fleischer K, van Wely M, Mastenbroek S. Preimplantation genetic testing for aneuploidies (abnormal number of chromosomes) in in vitro fertilisation. Cochrane Database Syst Rev. 2020;9:CD005291.
Leaver M, Wells D. Non-invasive preimplantation genetic testing (niPGT): the next revolution in reproductive genetics? Hum Reprod Update. 2020;26:16–42.
Hardy K, Warner A, Winston RM, Becker DL. Expression of intercellular junctions during preimplantation development of the human embryo. Mol Hum Reprod. 1996;2:621–32.
Daughtry BL, Chavez SL. Chromosomal instability in mammalian pre-implantation embryos: potential causes, detection methods, and clinical consequences. Cell Tissue Res. 2016;363:201–25.
Sadowy S, Ribustello L, Grossman LC, Guarnaccia M, Satriani K, Sauer MV. Cells excluded from development in human blastocysts are not representative of embryo ploidy. Fertil Steril. 2016;106:e17–8.
Armstrong S, Bhide P, Jordan V, Pacey A, Farquhar C. Time-lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database Syst Rev. 2018;5:CD011320.
Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373:2089–90.
Daughtry BL, Rosenkrantz JL, Lazar NH, Fei SS, Redmayne N, Torkenczy KA, et al. Single-cell sequencing of primate preimplantation embryos reveals chromosome elimination via cellular fragmentation and blastomere exclusion. Genome Res. 2019;29:367–82.
Lagalla C, Coticchio G, Sciajno R, Tarozzi N, Zaca C, Borini A. Alternative patterns of partial embryo compaction: prevalence, morphokinetic history and possible implications. Reprod Biomed Online. 2020;40:347–54.
Ebner T, Moser M, Shebl O, Sommergruber M, Gaiswinkler U, Tews G. Morphological analysis at compacting stage is a valuable prognostic tool for ICSI patients. Reprod Biomed Online. 2009;18:61–6.
Lagalla C, Tarozzi N, Sciajno R, Wells D, Di Santo M, Nadalini M, et al. Embryos with morphokinetic abnormalities may develop into euploid blastocysts. Reprod Biomed Online. 2017;34:137–46.
Coticchio G, Ezoe K, Lagalla C, Shimazaki K, Ohata K, Ninomiya M, et al. Perturbations of morphogenesis at the compaction stage affect blastocyst implantation and live birth rates. Hum Reprod. 2021;36:918–28.
Loewke K, Cho JH, Brumar CD, Maeder-York P, Barash O, Malmsten JE, et al. Characterization of an artificial intelligence model for ranking static images of blastocyst stage embryos. Fertil Steril. 2022;117:528–35.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
IRB of Mayo Clinic.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shenoy, C.C., Bader, A., Walker, D.L. et al. Embryo Blastomere Exclusion Identified in a Time-Lapse Culture System Is Associated with Embryo Ploidy. Reprod. Sci. 30, 1911–1916 (2023). https://doi.org/10.1007/s43032-022-01141-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01141-4