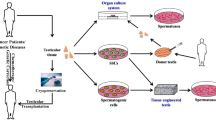
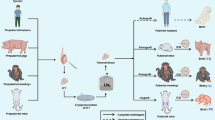
Abstract
Destruction of spermatogonial stem cells in juvenile men survivors of pediatric cancers leads to infertility as a side effect of gonadotoxic therapies. Sperm freezing before cancer treatment is commonly used in the clinic for fertility preservation, but this method is not applicable for prepubertal boys due to the lack of mature sperm. In these cases, cryopreservation of testicular tissues is the only option for fertility preservation. Although controlled slow freezing (CSF) is the most common procedure for testicular tissue cryopreservation, vitrification can be used as an alternative method. Controlled vitrification has prevented cell damage and formation of ice crystals. Procedures were done easily and quickly with a brief exposure time to high concentration of cryoprotectants without expensive equipment. Different studies used vitrification of testicular tissues and they assessed the morphology of seminiferous tubules, apoptosis, and viability of spermatogonial cells. Transplantation of vitrified testicular tissue into infertile recipient mice as well as in vitro culture of vitrified tissues was done in previous studies and their findings showed complete spermatogenesis and production of mature sperm. Review articles usually have compared controlled slow freezing with vitrification. In this review, we focused only on the vitrification method and its results. Despite promising results, many studies have been done for finding an optimal cryopreservation protocol in order to successfully preserve fertility in prepubertal boys.
Similar content being viewed by others
Code Availability
Not applicable.
References
Jang TH, et al. Cryopreservation and its clinical applications. Integr Med Res. 2017;6(1):12–8.
Liu X, et al. Male cancer patient sperm cryopreservation for fertility preservation: 10-year monocentric experience. Basic Clin Androl. 2021;31(1):1–9.
Aslam I, et al. Fertility preservation of boys undergoing anti-cancer therapy: A review of the existing situation and prospects for the future. Hum Reprod. 2000;15(10):2154–9.
Brenner H, Steliarova-Foucher E, Arndt V. Up-to-date monitoring of childhood cancer long-term survival in Europe: methodology and application to all forms of cancer combined. Ann Oncol. 2007;18(9):1561–8.
Benvenutti L, et al. Wistar rats immature testicular tissue vitrification and heterotopic grafting. JBRA Assist Reprod. 2018;22(3):167–73.
Trottmann M, et al. Semen quality in men with malignant diseases before and after therapy and the role of cryopreservation. Eur Urol. 2007;52(2):355–67.
Radaelli MRM, et al. A comparison between a new vitrification protocol and the slow freezing method in the cryopreservation of prepubertal testicular tissue. JBRA Assist Reprod. 2017;21(3):188–95.
Di Santo M, et al. Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv Urol. 2012.
Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316(5823):404–5.
Yango P, et al. Optimizing cryopreservation of human spermatogonial stem cells: comparing the effectiveness of testicular tissue and single cell suspension cryopreservation. Fertil Steril. 2014;102(5):1491-1498.e1.
Ogawa T, Ohmura M, Ohbo K. The niche for spermatogonial stem cells in the mammalian testis. Int J Hematol. 2005;82(5):381–8.
Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414(6859):98–104.
Gouk SS, et al. Cryopreservation of mouse testicular tissue: prospect for harvesting spermatogonial stem cells for fertility preservation. Fertil Steril. 2011;95(7):2399–403.
Keros V, et al. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22(5):1384–95.
Wyns C, et al. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum Reprod. 2011;26(4):737–47.
Dumont L, et al. Assessment of the optimal vitrification protocol for pre-pubertal mice testes leading to successful in vitro production of flagellated spermatozoa. Andrology. 2015;3(3):611–25.
Keros V, et al. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod. 2005;20(6):1676–87.
Kvist K, et al. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Hum Reprod. 2006;21(2):484–91.
Wyns C, et al. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Hum Reprod. 2007;22(6):1603–11.
Milazzo JP, et al. Comparison of conditions for cryopreservation of testicular tissue from immature mice. Hum Reprod. 2008;23(1):17–28.
Slabbert M. Investigating alternative sperm preservation methods for assisted reproductive technologies. 2013, University of Pretoria.
Pomeroy KO, et al. The ART of cryopreservation and its changing landscape. Fertil Steril. 2022;117(3):469–76.
Yokota Y, et al. Successful pregnancy following blastocyst vitrification: case report. Hum Reprod. 2000;15(8):1802–3.
Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature. 1985;313(6003):573–5.
Heo YS, et al. “Universal” vitrification of cells by ultra-fast cooling. Technology. 2015;3(01):64–71.
Yavin S, Arav A. Measurement of essential physical properties of vitrification solutions. Theriogenology. 2007;67(1):81–9.
Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17(3):243–56.
Zhang X, et al. Emerging technologies in medical applications of minimum volume vitrification. Nanomedicine (Lond). 2011;6(6):1115–29.
Sambu S. A Bayesian approach to optimizing cryopreservation protocols. PeerJ. 2015;3:e1039–e1039.
Shaw JM, Jones GM. Terminology associated with vitrification and other cryopreservation procedures for oocytes and embryos. Hum Reprod Update. 2003;9(6):583–605.
Karlsson JO. Cryopreservation: freezing and vitrification. Science. 2002;296(5568):655–6.
Yong KW, et al. Review of non-permeating cryoprotectants as supplements for vitrification of mammalian tissues. Cryobiology. 2020;96:1–11.
Ali J, Shelton JN. Design of vitrification solutions for the cryopreservation of embryos. J Reprod Fertil. 1993;99(2):471–7.
Fahy GM, et al. Vitrification as an approach to cryopreservation. Cryobiology. 1984;21(4):407–26.
Wusteman M, Robinson M, Pegg D. Vitrification of large tissues with dielectric warming: biological problems and some approaches to their solution. Cryobiology. 2004;48(2):179–89.
Baril G, et al. Successful direct transfer of vitrified sheep embryos. Theriogenology. 2001;56(2):299–305.
Song YC, et al. Vitreous preservation of articular cartilage grafts. J Invest Surg. 2004;17(2):65–70.
Jafarabadi M, Abdollahi M, Salehnia M. Assessment of vitrification outcome by xenotransplantation of ovarian cortex pieces in γ-irradiated mice: morphological and molecular analyses of apoptosis. J Assist Reprod Genet. 2015;32(2):195–205.
Curaba M, et al. Cryopreservation of prepubertal mouse testicular tissue by vitrification. Fertil Steril. 2011;95(4):1229-34 e1.
Amorim CA, et al. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod Biomed Online. 2011;23(2):160–86.
Quan GB, et al. Comparison of the effect of various disaccharides on frozen goat spermatozoa. Biopreserv Biobank. 2012;10(5):439–45.
Gosden RG. Gonadal tissue cryopreservation and transplantation. Reprod Biomed Online. 2002;4:64–7.
Hovatta O. Cryopreservation and culture of human ovarian cortical tissue containing early follicles. Eur J Obstet Gynecol Reprod Biol. 2004;113(Suppl 1):S50–4.
Fuller B, Paynter S. Fundamentals of cryobiology in reproductive medicine. Reprod Biomed Online. 2004;9(6):680–91.
Luyet BJ. The vitrification of organic colloids and of protoplasm. Biodynamica. 1937;29:1–14.
Gonzales F, Luyet B. Resumption of heart-beat in chick embryo frozen in liquid nitrogen. Biodynamica. 1950;7(126–128):1–5.
Farrant J. Mechanism of cell damage during freezing and thawing and its prevention. Nature. 1965;205(4978):1284–7.
Schiewe M, Anderson R. Vitrification: the pioneering past to current trends and perspectives of cryopreserving human embryos, gametes and reproductive tissue. J Biorepository Sci Appl Med. 2017;5:57–68.
Rapatz G, Luyet B. Electron microscope study of erythrocytes in rapidly cooled suspensions containing various concentrations of glycerol. Biodynamica. 1968;10(210):193–210.
Boutron P, Kaufmann A. Stability of the amorphous state in the system water-glycerol-dimethylsulfoxide. Cryobiology. 1978;15(1):93–108.
Fahy GM, et al. Physical and biological aspects of renal vitrification. Organogenesis. 2009;5(3):167–75.
Finger EB, Bischof JC. Cryopreservation by vitrification: a promising approach for transplant organ banking. Curr Opin Organ Transplant. 2018;23(3):353–60.
Shaw JM, Diotallevi L, Trounson AO. A simple rapid 4–5 m dimethyl-sulfoxide freezing technique for the cryopreservation of one-cell to blastocyst stage preimplantation mouse embryos. Reprod Fertil Dev. 1991;3(5):621–6.
Shaw JM, et al. Vitrification properties of solutions of ethylene glycol in saline containing PVP, Ficoll, or dextran. Cryobiology. 1997;35(3):219–29.
Mukaida T, et al. Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Hum Reprod. 1998;13(10):2874–9.
Vajta G, et al. Open pulled straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev:Inc Gamete Res. 1998;51(1):53–8.
Kuleshova L, et al. Birth following vitrification of a small number of human oocytes. Hum Reprod. 1999;14(12):3077–9.
Cobo A, et al. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod. 2010;25(9):2239–46.
Zhu D, et al. Vitrified-warmed blastocyst transfer cycles yield higher pregnancy and implantation rates compared with fresh blastocyst transfer cycles - time for a new embryo transfer strategy? Fertil Steril. 2011;95(5):1691–5.
Chang C-C, Huang J-C, Shen P-C. Method for microdrop vitrification of cells. 2006, Google Patents.
Xing W, et al. Solid-surface vitrification is an appropriate and convenient method for cryopreservation of isolated rat follicles. Reprod Biol Endocrinol. 2010;8(1):1–9.
Seki S, Mazur P. Ultra-rapid warming yields high survival of mouse oocytes cooled to -196°c in dilutions of a standard vitrification solution. PLoS One. 2012;7(4).
Mazur P, Seki S. Survival of mouse oocytes after being cooled in a vitrification solution to -196°C at 95° to 70,000°C/min and warmed at 610° to 118,000°C/min: a new paradigm for cryopreservation by vitrification. Cryobiology. 2011;62(1):1–7.
Gutnisky C, et al. Evaluation of the Cryotech Vitrification Kit for bovine embryos. Cryobiology. 2013;67(3):391–3.
Allahbadia GN, Gandhi G. Vitrification in assisted reproduction. 2016: Springer.
Fahy GM, et al. Cryopreservation of organs by vitrification: perspectives and recent advances. Cryobiology. 2004;48(2):157–78.
Armstrong AG, et al. Ovarian tissue cryopreservation in young females through the Oncofertility Consortium’s National Physicians Cooperative. Future Oncol. 2018;14(4):363–78.
Liebermann J, et al. Potential importance of vitrification in reproductive medicine. Biol Reprod. 2002;67(6):1671–80.
Kader AA, et al. Factors affecting the outcome of human blastocyst vitrification. Reprod Biol Endocrinol. 2009;7:99.
Cho HJ, et al. An improved protocol for dilution of cryoprotectants from vitrified human blastocysts. Hum Reprod. 2002;17(9):2419–22.
Poels J, et al. Vitrification of non-human primate immature testicular tissue allows maintenance of proliferating spermatogonial cells after xenografting to recipient mice. Theriogenology. 2012;77(5):1008–13.
Baert Y, et al. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertil Steril. 2012;97(5):1152-1157.e2.
Liu I, Cheng KM, Silversides FG. Production of live offspring from testicular tissue cryopreserved by vitrification procedures in Japanese quail (Coturnix japonica). Biol Reprod. 2013;88(5).
Chatdarong K, Thuwanut P, Morrell JM. The development of cat testicular sperm cryopreservation protocols: effects of tissue fragments or sperm cell suspension. Theriogenology. 2016;85(2):200–6.
Curaba M, et al. Can prepubertal human testicular tissue be cryopreserved by vitrification? Fertil Steril. 2011;95(6):2123 e9–12.
Baert Y, et al. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod. 2013;28(7):1816–26.
Poels J, et al. Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod. 2013;28(3):578–89.
Sa R, et al. Cryopreservation of human testicular diploid germ cell suspensions. Andrologia. 2012;44(6):366–72.
Pegg DE. The role of vitrification techniques of cryopreservation in reproductive medicine. Hum Fertil. 2005;8(4):231–9.
Abrishami M, et al. Cryopreservation of immature porcine testis tissue to maintain its developmental potential after xenografting into recipient mice. Theriogenology. 2010;73(1):86–96.
Kaneko H, et al. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS One. 2013;8(7):e70989.
Yokonishi T, et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun. 2014;5:4320.
Bhakta G, et al. Cryoreservation of alginate-fibrin beads involving bone marrow derived mesenchymal stromal cells by vitrification. Biomaterials. 2009;30(3):336–43.
Kuleshova LL, et al. Vitrification of encapsulated hepatocytes with reduced cooling/warming rates. Cryo-Letters. 2004;25(4):241–54.
Tan FCK, et al. Optimization of cryopreservation of stem cells cultured as neurospheres: comparison between vitrification, slow-cooling and raped cooling “freezing” protocols. Cryo-Letters. 2007;28(6):445–60.
Wen F, et al. Vitreous cryopreservation of nanofibrous tissue-engineered constructs generated using mesenchymal stromal cells. Tissue Eng-Part C: Methods. 2009;15(1):105–14.
Martincic DS, et al. Germ cell apoptosis in the human testis. Pflugers Arch. 2001;442(6 Suppl 1):R159–60.
Aggarwal A, et al. Adverse effects associated with persistent stimulation of Leydig cells with hCG in vitro. Mol Reprod Dev. 2009;76(11):1076–83.
Nagata S, Golstein P. The Fas death factor. Science. 1995;267(5203):1449–56.
Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–56.
Shaha C, Tripathi R, Mishra DP. Male germ cell apoptosis: regulation and biology. Philos Trans R Soc Lond B Biol Sci. 2010;365(1546):1501–15.
Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2(4):277–88.
Majno G, Joris I. Apoptosis, oncosis, and necrosis an overview of cell death. Am J Pathol. 1995;146(1):3–15.
Papaliagkas V, et al. The proteins and the mechanisms of apoptosis: a mini-review of the fundamentals. Hippokratia. 2007;11(3):108–13.
Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148(7):2207–16.
Aitken RJ, et al. Apoptosis in the germ line. Reproduction. 2011;141(2):139–50.
Soussi T, Caron de Fromentel C, May P. Structural aspects of the p53 protein in relation to gene evolution. Oncogene, 1990;5(7):945–52.
Hemadi M, et al. Assessment of morphological and functional changes in neonate vitrified testis grafts after host treatment with melatonin. Folia Morphol (Warsz). 2011;70(2):95–102.
Hemadi M, et al. Potential use of melatonin supplementation to protect vitrified testicular grafts from hypoxic-ischaemic damage. Andrologia. 2014;46(5):513–21.
Gholami M, et al. Does prepubertal testicular tissue vitrification influence spermatogonial stem cells (SSCs) viability? J Assist Reprod Genet. 2013;30(10):1271–7.
Hajiaghalou S, et al. Comparison of apoptosis pathway following the use of two protocols for vitrification of immature mouse testicular tissue. Theriogenology. 2016;86(8):2073–82.
Dumont L, et al. Evaluation of apoptotic- and autophagic-related protein expressions before and after IVM of fresh, slow-frozen and vitrified pre-pubertal mouse testicular tissue. Mol Hum Reprod. 2017;23(11):738–54.
Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011;119(1):3–19.
Bebbere D, et al. Gene expression analysis of ovine prepubertal testicular tissue vitrified with a novel cryodevice (E.Vit). J Assist Reprod Genet. 2019;36(10):2145–54.
Oblette A, et al. DNA methylation and histone post-translational modifications in the mouse germline following in-vitro maturation of fresh or cryopreserved prepubertal testicular tissue. Reprod Biomed Online. 2019;39(3):383–401.
Jahnukainen K, Mitchell RT, Stukenborg JB. Testicular function and fertility preservation after treatment for haematological cancer. Curr Opin Endocrinol Diabetes Obes. 2015;22(3):217–23.
Patience C, Takeuchi Y, Weiss RA. Zoonosis in xenotransplantation. Curr Opin Immunol. 1998;10(5):539–42.
Abu Elhija M, et al. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J Androl. 2012;14(2):285–93.
Arkoun B, et al. Does soaking temperature during controlled slow freezing of pre-pubertal mouse testes influence course of in vitro spermatogenesis? Cell Tissue Res. 2016;364(3):661–74.
Dumont L, et al. Vitamin A prevents round spermatid nuclear damage and promotes the production of motile sperm during in vitro maturation of vitrified pre-pubertal mouse testicular tissue. Mol Hum Reprod. 2016;22(12):819–32.
Rondanino C, et al. Establishment, maintenance and functional integrity of the blood-testis barrier in organotypic cultures of fresh and frozen/thawed prepubertal mouse testes. Mol Hum Reprod. 2017;23(5):304–20.
Sato T, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471(7339):504–7.
Lima DBC, Silva L, Comizzoli P. Influence of warming and reanimation conditions on seminiferous tubule morphology, mitochondrial activity, and cell composition of vitrified testicular tissues in the domestic cat model. PLoS One. 2018;13(11):e0207317.
Woods EJ, et al. Fundamental cryobiology of reproductive cells and tissues. Cryobiology. 2004;48(2):146–56.
Yildiz C, et al. Comparison of cryosurvival and spermatogenesis efficiency of cryopreserved neonatal mouse testicular tissue between three vitrification protocols and controlled-rate freezing. Cryobiology. 2018;84:4–9.
Kimura Y, Yanagimachi R. Mouse oocytes injected with testicular spermatozoa or round spermatids can develop into normal offspring. Development. 1995;121(8):2397–405.
Gilmore JA, et al. Determination of optimal cryoprotectants and procedures for their addition and removal from human spermatozoa. Hum Reprod. 1997;12(1):112–8.
Kaneko H, et al. Production of sperm from porcine fetal testicular tissue after cryopreservation and grafting into nude mice. Theriogenology. 2017;91:154–62.
Pukazhenthi BS, et al. Slow freezing, but not vitrification supports complete spermatogenesis in cryopreserved, neonatal sheep testicular xenografts. PLoS One. 2015;10(4):e0123957.
Yamini N, et al. Developmental potential of vitrified mouse testicular tissue after ectopic transplantation. Cell J. 2016;18(1):74–82.
Poels J, et al. In search of better spermatogonial preservation by supplementation of cryopreserved human immature testicular tissue xenografts with N-acetylcysteine and testosterone. Front Surg. 2014;1:47.
Wu B, et al. iNOS enhances rat intestinal apoptosis after ischemia-reperfusion. Free Radic Biol Med. 2002;33(5):649–58.
Frederickx V, et al. Recovery, survival and functional evaluation by transplantation of frozen–thawed mouse germ cells. Hum Reprod. 2004;19(4):948–53.
Israely T, et al. Reducing ischaemic damage in rodent ovarian xenografts transplanted into granulation tissue. Hum Reprod. 2006;21(6):1368–79.
Wyns C, et al. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum Reprod. 2008;23(11):2402–14.
Rathi R, et al. Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology. 2008;149(10):5288–96.
Schlatt S, et al. Modulating testicular mass in xenografting: a model to explore testis development and endocrine function. Endocrinology. 2010;151(8):4018–23.
Zeng W, et al. The length of the spermatogenic cycle is conserved in porcine and ovine testis xenografts. J Androl. 2006;27(4):527–33.
Pietzak EJ 3rd, et al. Histology of testicular biopsies obtained for experimental fertility preservation protocol in boys with cancer. J Urol. 2015;194(5):1420–4.
Kilcoyne KR, Mitchell RT. Fertility preservation: testicular transplantation for fertility preservation: clinical potential and current challenges. Reproduction. 2019;158(5):F1-f14.
Onofre J, et al. Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum Reprod Update. 2016;22(6):744–61.
Tang W, et al. Up-regulation of heme oxygenase-1 expression modulates reactive oxygen species level during the cryopreservation of human seminiferous tubules. Fertil Steril. 2014;102(4):974-980 e4.
Nagy ZP, Shapiro D, Chang CC. Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril. 2020;113(2):241–7.
Yokonishi T, et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun. 2014;5(1):1–6.
Shinohara T, et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod. 2002;17(12):3039–45.
Acknowledgements
We would like to thank the Anatomical Department of Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This was a review article on existing literature and did not need review by the institutional ethics committee.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nikmahzar, A., Khadivi, F., Abbasi, M. et al. Testicular Tissue Vitrification: a Promising Strategy for Male Fertility Preservation. Reprod. Sci. 30, 1687–1700 (2023). https://doi.org/10.1007/s43032-022-01113-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01113-8