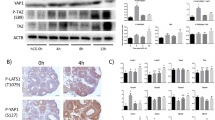
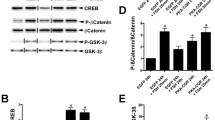
Abstract
Granulosa cells (GCs) must respond appropriately to follicle-stimulating hormone (FSH) for proper follicle maturation. FSH activates protein kinase A (PKA) leading to phosphorylation of the cyclic AMP response element binding protein-1 (CREB1). We identified a unique A-kinase anchoring protein (AKAP13) containing a Rho guanine nucleotide exchange factor (RhoGEF) region that was induced in GCs during folliculogenesis. AKAPs are known to coordinate signaling cascades, and we sought to evaluate the role of AKAP13 in GCs in response to FSH. Aromatase reporter activity was increased in COV434 human GCs overexpressing AKAP13. Addition of FSH, or the PKA activator forskolin, significantly enhanced this activity by 1.5- to 2.5-fold, respectively (p < 0.001). Treatment with the PKA inhibitor H89 significantly reduced AKAP13-dependent activation of an aromatase reporter (p = 0.0067). AKAP13 physically interacted with CREB1 in co-immunoprecipitation experiments and increased the phosphorylation of CREB1. CREB1 phosphorylation increased after FSH treatment in a time-specific manner, and this effect was reduced by siRNA directed against AKAP13 (p = 0.05). CREB1 activation increased by 18.5-fold with co-expression of AKAP13 in the presence of FSH (p < 0.001). Aromatase reporter activity was reduced by inhibitors of the RhoGEF region, C3 transferase and A13, and greatly enhanced by the RhoGEF activator, A02. In primary murine and COV43 GCs, siRNA knockdown of Akap13/AKAP13 decreased aromatase and luteinizing hormone receptor transcripts in cells treated with FSH, compared with controls. Collectively, these findings suggest that AKAP13 may function as a scaffolding protein in FSH signal transduction via an interaction with CREB, resulting in phosphorylation of CREB.





Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: Branching out from protein kinase A. Cell Signal. 2006;18(9):1351–9. https://doi.org/10.1016/j.cellsig.2006.02.011.
Law NC, Weck J, Kyriss B, Nilson JH, Hunzicker-Dunn M. Lhcgr Expression in granulosa cells: roles for PKA-phosphorylated β-catenin, TCF3, and FOXO1. Mol Endocrinol. 2013;27(8):1295–310. https://doi.org/10.1210/me.2013-1025.
Escamilla-Hernandez R, Little-Ihrig L, Orwig KE, Yue J, Chandran U, Zeleznik AJ. Constitutively active protein kinase A qualitatively mimics the effects of follicle-stimulating hormone on granulosa cell differentiation. Mol Endocrinol. 2008;22(8):1842–52. https://doi.org/10.1210/me.2008-0103.
Puri P, Little-Ihrig L, Chandran U, Law NC, Hunzicker-Dunn M, Zeleznik AJ. Protein kinase A a master kinase of granulosa cell differentiation. Sci Rep. 2016;6(1):28132. https://doi.org/10.1038/srep28132.
Vassart G, Smits G, Campillo M, et al. Glycoprotein hormone receptors: determinants in leucine-rich repeats responsible for ligand specificity. EMBO J. 2003;22(11):2692–703. https://doi.org/10.1093/emboj/cdg260.
Beebe SJ, Segaloff DL, Burks D, Beasley-Leach A, Limbird LE, Corbin JD. Evidence that cyclic adenosine 3’,5’-monophosphate-dependent protein kinase activation causes pig ovarian granulosa cell differentiation, including increases in two type II subclasses of this kinase. Biol Reprod. 1989;41(2):295–307. https://doi.org/10.1095/biolreprod41.2.295.
DeManno DA, Cottom JE, Kline MP, Peters CA, Maizels ET, Hunzicker-Dunn M. Follicle-stimulating hormone promotes histone H3 phosphorylation on serine-10. Mol Endocrinol. 1999;13(1):91–105. https://doi.org/10.1210/mend.13.1.0222.
Hillier SG, Zeleznik AJ, Ross GT. Independence of steroidogenic capacity and luteinizing hormone receptor induction in developing granulosa cells. Endocrinology. 1978;102(3):937–46. https://doi.org/10.1210/endo-102-3-937.
Welch EJ, Jones BW, Scott JD. Networking with AKAPs: context-dependent regulation of anchored enzymes. Mol Interv. 2010;10(2):86–97. https://doi.org/10.1124/mi.10.2.6.
Skalhegg BS, Tasken K. Specificity in the cAMP PKA signaling pathway differential expression regulation and subcellular localization of subunits of PKA. Front Biosci. 2000;5(3):678. https://doi.org/10.2741/A543.
Oyên O, Myklebust F, Scott JD, et al. Subunits of cyclic adenosine 3’,5’-monophosphate-dependent protein kinase show differential and distinct expression patterns during germ cell differentiation: alternative polyadenylation in germ cells gives rise to unique smaller-sized mRNA species. Biol Reprod. 1990;43(1):46–54. https://doi.org/10.1095/biolreprod43.1.46.
Richards JS, Haddox M, Tash JS, Walter U, Lohmann S. Adenosine 3′,5′-monophosphate-dependent protein kinase and granulosa cell responsiveness to gonadotropins. Endocrinology. 1984;114(6):2190–8. https://doi.org/10.1210/endo-114-6-2190.
Ratoosh SL, Richards JS. Regulation of the content and phosphorylation of RII by adenosine 3’,5’-monophosphate, follicle-stimulating hormone, and estradiol in cultured granulosa cells. Endocrinology. 1985;117(3):917–27. https://doi.org/10.1210/endo-117-3-917.
Newhall KJ, Criniti AR, Cheah CS, et al. Dynamic anchoring of PKA is essential during oocyte maturation. Curr Biol. 2006;16(3):321–7. https://doi.org/10.1016/j.cub.2005.12.031.
Carlone DL, Richards JS. Evidence that functional interactions of CREB and SF-1 mediate hormone regulated expression of the aromatase gene in granulosa cells and constitutive expression in R2C cells. J Steroid Biochem Mol Biol. 1997;61(3):223–31. https://doi.org/10.1016/S0960-0760(96)00206-3.
Conti M. Specificity of the cyclic adenosine 3′,5′-monophosphate signal in granulosa cell function. Biol Reprod. 2002;67(6):1653–61. https://doi.org/10.1095/biolreprod.102.004952.
Maizels ET. Follicle Stimulating Hormone (FSH) Activates the p38 mitogen-activated protein kinase pathway, inducing small heat shock protein phosphorylation and cell rounding in immature rat ovarian granulosa cells. Endocrinology. 1998;139(7):3353–6. https://doi.org/10.1210/en.139.7.3353.
Wayne CM, Fan H, Cheng X, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol. 2007;21(8):1940–57. https://doi.org/10.1210/me.2007-0020.
Cottom J, Salvador LM, Maizels ET, et al. Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa Phosphotyrosine Phosphatase. J Biol Chem. 2003;278(9):7167–79. https://doi.org/10.1074/jbc.M203901200.
Rubino D, Driggers P, Arbit D, et al. Characterization of Brx, a novel Dbl family member that modulates estrogen receptor action. Oncogene. 1998;16(19):2513–26. https://doi.org/10.1038/sj.onc.1201783.
Scott JD, Wong W. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5(12):959–70. https://doi.org/10.1038/nrm1527.
Feliciello A, Gottesman ME, Avvedimento EV. The biological functions of A-kinase anchor proteins. J Mol Biol. 2001;308(2):99–114. https://doi.org/10.1006/jmbi.2001.4585.
Zheng Y, Olson MF, Hall A, Cerione RA, Toksoz D. Direct involvement of the small GTP-binding protein Rho in lbc oncogene function. J Biol Chem. 1995;270(16):9031–4. https://doi.org/10.1074/jbc.270.16.9031.
Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Galpha 12-selective Rho-mediated stress fiber formation. J Biol Chem. 2001;276(47):44247–57. https://doi.org/10.1074/jbc.M106629200.
Driggers PH, Segars JH, Rubino DM. The proto-oncoprotein Brx activates estrogen receptor β by a p38 mitogen-activated protein kinase pathway. J Biol Chem. 2001;276(50):46792–7. https://doi.org/10.1074/jbc.M106927200.
Kino T, Souvatzoglou E, Charmandari E, et al. Rho family guanine nucleotide exchange factor Brx couples extracellular signals to the glucocorticoid signaling system. J Biol Chem. 2006;281(14):9118–26. https://doi.org/10.1074/jbc.M509339200.
Ng SS, Jorge S, Malik M, et al. A-Kinase Anchoring Protein 13 (AKAP13) Augments progesterone signaling in uterine fibroid cells. J Clin Endocrinol Metab. 2019;104(3):970–80. https://doi.org/10.1210/jc.2018-01216.
Maravet Baig K, Su S, Mumford SL, et al. Mice deficient in AKAP13 (BRX) develop compulsive-like behavior and increased body weight. Brain Res Bull. 2018;140:72–9. https://doi.org/10.1016/j.brainresbull.2018.04.005.
Koide H, Holmbeck K, Lui JC, et al. Mice deficient in AKAP13 (BRX) are osteoporotic and have impaired osteogenesis. J Bone Miner Res. 2015;30(10):1887–95. https://doi.org/10.1002/jbmr.2534.
Ohgushi M, Minaguchi M, Sasai Y. Rho-signaling-directed YAP/TAZ activity underlies the long-term survival and expansion of human embryonic stem cells. Cell Stem Cell. 2015;17(4):448–61. https://doi.org/10.1016/j.stem.2015.07.009.
Qiao Z, Jiang Y, Wang L, Wang L, Jiang J, Zhang J. Mutations in KIAA1109, CACNA1C, BSN, AKAP13, CELSR2, and HELZ2 are associated with the prognosis in endometrial cancer. Front Genet. 2019;10:909. https://doi.org/10.3389/fgene.2019.00909.
Miller BT, Rubino DM, Driggers PH, et al. Expression of brx proto-oncogene in normal ovary and in epithelial ovarian neoplasms. Am J Obstet Gynecol. 2000;182(2):286–95. https://doi.org/10.1016/S0002-9378(00)70213-4.
Hearns-Stokes R, Mayers C, Zahn C, et al. Expression of the proto-oncoprotein breast cancer nuclear receptor auxiliary factor (Brx) is altered in eutopic endometrium of women with endometriosis. Fertil Steril. 2006;85(1):63–70. https://doi.org/10.1016/j.fertnstert.2005.06.053.
Alam H, Maizels ET, Park Y, et al. Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem. 2004;279(19):19431–40. https://doi.org/10.1074/jbc.M401235200.
Carr DW, DeManno DA, Atwood A, Hunzicker-Dunn M, Scott JD. Follicle-stimulating hormone regulation of A-kinase anchoring proteins in granulosa cells. J Biol Chem. 1993;268(28):20729–32. https://doi.org/10.1016/S0021-9258(19)36841-3.
Gong X, McGee EA. Smad3 is required for normal follicular follicle-stimulating hormone responsiveness in the mouse. Biol Reprod. 2009;81(4):730–8. https://doi.org/10.1095/biolreprod.108.070086.
van den Berg-Bakker Cornelia A. M, Hagemeijer A, Franken-Postma EM, et al. Establishment and characterization of 7 ovarian carcinoma cell lines and one granulosa tumor cell line: Growth features and cytogenetics. Int J Cancer. 1993;53(4):613–20. https://doi.org/10.1002/ijc.2910530415.
Zhang H, Vollmer M, De Geyter M, et al. Characterization of an immortalized human granulosa cell line (COV434). Mol Hum Reprod. 2000;6(2):146–53. https://doi.org/10.1093/molehr/6.2.146.
Diviani D, Raimondi F, Del Vescovo C, et al. Small-molecule protein-protein interaction inhibitor of oncogenic Rho signaling. Cell Chem Biol. 2016;23(9):1135–46. https://doi.org/10.1016/j.chembiol.2016.07.015.
Mayers CM, Wadell J, McLean K, et al. The Rho guanine nucleotide exchange factor AKAP13 (BRX) is essential for cardiac development in mice. J Biol Chem. 2010;285(16):12344–54. https://doi.org/10.1074/jbc.M110.106856.
Maher JY, Islam MS, Yin O, et al. The role of Hippo pathway signaling and A-kinase anchoring protein 13 in primordial follicle activation and inhibition. F&S Science. 2022;3(2):118–29. https://doi.org/10.1016/j.xfss.2022.03.002.
Zhang X, Yuan R, Bai Y, Yang Y, Song X, Lan X, Pan C. A deletion mutation within the goat AKAP13 gene is significantly associated with litter size. Anim Biotechnol. 2021. https://doi.org/10.1080/10495398.2021.1968418.
Zhang P, Wang J, Lang H, et al. Knockdown of CREB1 promotes apoptosis and decreases estradiol synthesis in mouse granulosa cells. Biomed Pharmacother. 2018;105:1141–6. https://doi.org/10.1016/j.biopha.2018.06.101.
Carr DW, Cutler JRE, Cottom JE, et al. Identification of cAMP-dependent protein kinase holoenzymes in preantral- and preovulatory-follicle-enriched ovaries, and their association with A-kinase-anchoring proteins. Biochem J. 1999;344(2):613–23. https://doi.org/10.1042/0264-6021:3440613.
Gu Y, Xu W, Zhuang B, Fu W. Role of A-kinase anchoring protein 95 in the regulation of cytochrome P450 family 19 subfamily A member 1 (CYP19A1) in human ovarian granulosa cells. Reprod Fertil Dev. 2018;30(8):1128–36. https://doi.org/10.1071/RD17313.
Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15(3):510–9. https://doi.org/10.1002/j.1460-2075.1996.tb00383.x.
Ellerbroek SM, Wennerberg K, Burridge K. Serine phosphorylation negatively regulates RhoA in vivo. The J Biol Chem. 2003;278(21):19023–31. https://doi.org/10.1074/jbc.M213066200.
Diviani D, Abuin L, Cotecchia S, Pansier L. Anchoring of both PKA and 14–3–3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO J. 2004;23(14):2811–20. https://doi.org/10.1038/sj.emboj.7600287.
Lang P, Gesbert F, Thiberge JM, et al. Characterization of a monoclonal antibody specific for the Ras-related GTP-bBinding protein Rho A. Biochem Biophys Res Commun. 1993;196(3):1522–8. https://doi.org/10.1006/bbrc.1993.2424.
Salvador LM, Flynn MP, Avila J, et al. Neuronal microtubule-associated protein 2D is a dual A-kinase anchoring protein expressed in rat ovarian granulosa cells. The J Biol Chem. 2004;279(26):27621–32. https://doi.org/10.1074/jbc.M402980200.
Kawamura K, Cheng Y, Suzuki N, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA. 2013;110(43):17474–9. https://doi.org/10.1073/pnas.1312830110.
Li T, Zhao H, Zhao X, et al. Identification of YAP1 as a novel susceptibility gene for polycystic ovary syndrome. J Med Genet. 2012;49(4):254–7. https://doi.org/10.1136/jmedgenet-2011-100727.
Acknowledgements
The authors thank Joshua T. Brennan, M.S., MPH and Md Soriful Islam, Ph.D.
Funding
Research reported in this publication was supported in part by the Howard and Georgeanna Jones Endowment; National Institutes of Health Grant ZIA-HD-008737–11 (to J.H.S.); the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K12-HD-103036 (to K.C.V); the Society for Reproductive Investigation and Bayer Discovery Innovation Grant (to K.C.V); the Clinical Research Training Program, a public–private partnership supported jointly by the National Institutes of Health (to K.D. and M.M.); the Johns Hopkins University School of Medicine Predoctoral Research Program for Medical Students (Dean’s Year of Research) (to A.S.C.); NIH ZIA-HD-008985 (to J.Y.M.); and the Edward E. Wallach Research Award (to J.Y.M.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kamaria C. Cayton Vaught, Kate Devine, Ashlie Sewdass, Jacqueline Y. Maher, Dana Hazimeh, Marcy Maguire, Elizabeth A. McGee, Paul H. Driggers, and James H. Segars. The first draft of the manuscript was written by Kamaria C. Cayton Vaught and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experimental procedures involving animals were conducted in accordance with the Intuitional Animal Care and Use Committees at the National Institute of Health. Animals were cared for as per institutional regulations under the reviewed and approved animal study protocols, ASP 03–001, ASP 06–002, ASP 12–060, and ASP 15–060.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cayton Vaught, K.C., Hazimeh, D., Carter, A.S. et al. AKAP13 Enhances CREB1 Activation by FSH in Granulosa Cells. Reprod. Sci. 30, 1528–1539 (2023). https://doi.org/10.1007/s43032-022-01097-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01097-5