Abstract
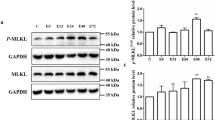
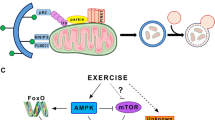
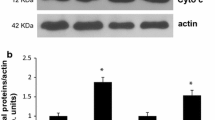
High mobility group box-1 protein (HMGB1) is an evolutionarily ancient protein, which, as an important non-histone chromosome-binding protein in organism cells, is involved in a variety of important biological processes, including DNA repair, gene transcription, cellular inflammatory response, and autophagy. In this study, we established an eccentric exercise model to observe the effect of HMGB1 on skeletal muscle autophagy and to investigate the underlying molecular mechanisms. Forty-eight male 8-week-old SD rats were randomly divided into control group (C) and exercise group (E). Group E was subjected to a bout of eccentric exercise on a treadmill and sampled soleus at 0 h, 12 h, 24 h, 48 h, and 72 h post-exercise. The speed of the exercise protocol in this study was 16 m/min, the slope was −16°, and the time was 90 min. The ultrastructural changes of skeletal muscle were observed by transmission electron microscopy. The protein expressions of HMGB1, Beclin1, and LC3 were detected by Western Blot. The co-localizations of Beclin1/Bcl-2, Beclin1/HMGB1, and Beclin1/Vps34 were measured by immunofluorescence. The results show that eccentric exercise leads to abnormal changes in the ultrastructure of skeletal muscle, and the protein levels of Beclin1, LC3-II/LC3-I, and the content of HMGB1 in nuclear and cytoplasm were significantly increased at 24 h post-exercise (P < 0.05). The co-localization of Beclin1/Bcl-2 and Beclin1/HMGB1 were increased significantly at 0 h post-exercise and then decreased, while the co-localization of Beclin1/Vps34 showed the highest level at 24 h post-exercise. In conclusion, HMGB1 facilitates the separation of Beclin1 from Bcl-2 and promotes Beclin1 binding to Vps34, which may play an important role in eccentric exercise-induced skeletal muscle autophagy.








Similar content being viewed by others
Data Availability
We confirm that we have included a data availability statement in my main manuscript file: “The role of autophagy regulator HMGB1 in skeletal muscle autophagy after eccentric exercise.”
References
Boecker CA, Holzbaur EL. Vesicular degradation pathways in neurons: at the crossroads of autophagy and endo-lysosomal degradation. Curr Opin Neurobiol. 2019;57:94–101.
Chang NC, Nguyen M, Germain M, Shore GC. Antagonism of Beclin 1-dependent autophagy by BCL-2 at the endoplasmic reticulum requires NAF-1. EMBO J. 2010;29(3):606–18.
Dhar SK, Bakthavatchalu V, Dhar B, Chen J, Tadahide I, Zhu H, Gao T, St Clair DK. DNA polymerase gamma (Polgamma) deficiency triggers a selective mTORC2 prosurvival autophagy response via mitochondria-mediated ROS signaling. Oncogene. 2018;37(48):6225–42.
Dong XD, Ito N, Lotze MT, DeMarco WA, Popovic P, Shand SH, Watkins S, Winikoff S, Brown CK, Bartlett DL, Zeh HJ. High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother. 2007;30(6):596–606.
Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Volpi E, Rasmussen BB. Skeletal muscle autophagy and protein breakdown following resistance exercise are similar in younger and older adults. J Gerontol A Biol Sci Med Sci. 2013;68(5):599–607. https://doi.org/10.1093/gerona/gls209.
Hjert CS, Wright CJ. The effects of self-myofascial release foam rolling on muscle soreness or pain after experiencing delayed onset muscle soreness: a critically appraised topic. Int J Athl Therapy Train. 2020;25(6):294–8.
Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8(4):445–544. https://doi.org/10.4161/auto.19496.
Kumar S, Jain A, Choi SW, Duarte da Silva GP, Allers L, Mudd MH, Peters RS, Anonsen JH, Rusten TE, Lazarou M, Deretic V. Mammalian Atg8-family proteins are upstream regulators of the lysosomalsystem by controlling MTOR and TFEB. Autophagy. 2020;16(12):2305–6.
Lei RH, Yan LB, Deng YL, Xu J, Zhao T, Awan MUF, Li Q, Zhou GM, Wang X, Ma H. HMGB1 mediated autophagy protects glioblastoma cells from carbon-ion beam irradiation injury. Acta Astronaut. 2020;166:628–34.
Li YY, Xie JW, Li XY, Fang JP. Poly (ADP-ribosylation) of HMGB1 facilitates its acetylation and promotes HMGB1 translocation-associated chemotherapy-induced autophagy in leukaemia cells. Oncol Lett. 2020;19(1):368–78.
Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a Novel Bcl-2-interacting protein. J Virol. 1998;72(11):8586–96.
Liu Y, Yan W, Tohme S, Chen M, Fu Y, Tian D, Lotze M, Tang D, Tsung A. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J Hepatol. 2015;63(1):114–21.
Marquez RT, Xu L. Bcl-2: Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res. 2012;2(2):214–21.
Meng YC, Lou XL, Yang LY, Li D, Hou YQ. Role of the autophagy-related marker LC3 expression in hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol. 2020;146(5):1103–13.
Nichenko AS, Southern WM, Tehrani KF, Yin A, Mortensen LJ, Yin H, Call JA. Mitochondrial-specific autophagy linked to mitochondrial dysfunction following traumatic freeze injury in mice. Am J Physiol Cell Physiol. 2020;318(2):C242–52. https://doi.org/10.1152/ajpcell.00123.2019.
Owens DJ, Twist C, Cobley JN, Howatson G, Close GL. Exercise-induced muscle damage: what is it, what causes it and what are the nutritional solutions? Eur J Sport Sci. 2019;19(1):71–85.
Rochman M, Postnikov Y, Correll S, Malicet C, Wincovitch S, Karpova TS, McNally JG, Wu X, Bubunenko NA, Grigoryev S, Bustin M. The interaction of NSBP1/HMGN5 with nucleosomes in euchromatin counteracts linker histone-mediated chromatin compaction and modulates transcription. Mol Cell. 2009;35:642–56.
Salminen A, Vihko V. Autophagic response to strenuous exercise in mouse skeletal muscle fibers. Virch Archiv B Cell Pathol. 1984;45(1):97–106. https://doi.org/10.1007/BF02889856.
Shang H, Xia Z, Bai S, Zhang HE, Gu B, Wang R. Downhill running acutely elicits mitophagy in rat soleus muscle. Med Sci Sports Exerc. 2019;51(7):1396–403.
Strappazzon F, Vietri-Rudan M, Campello S, Nazio F, Florenzano F, Fimia GM, Piacentini M, Levine B, Cecconi F. Mitochondrial BCL-2 inhibits AMBRA1-induced autophagy. EMBO J. 2011;30(7):1195–208.
Tan Q, Wang M, Yu M, Zhang J, Bristow RG, Hill RP, Tannock IF. Role of autophagy as a survival mechanism for hypoxic cells in tumors. Neoplasia. 2016;18(6):347–55.
Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ 3rd, Lotze MT. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190(5):881–92.
Ugrinova I, Pasheva E. HMGB1 protein: a therapeutic target inside and outside the cell. Adv Protein Chem Struct Biol. 2017;107:37–76.
Vergoten G, Bailly C. N-glycosylation of high mobility group box 1 protein (HMGB1) modulates the interaction with glycyrrhizin: a molecular modeling study. Comput Biol Chem. 2020;88:107312.
Wang K, Huang J, Xie W, Huang L, Zhong C, Chen Z. Beclin1 and HMGB1 ameliorate the alpha-synuclein-mediated autophagy inhibition in PC12 cells. Diagn Pathol. 2016;11:15.
Webster Anthony L, Syrotuik Daniel G, Bell Gordon J, Jones Richard L, Hanstock CC. Effects of hyperbaric oxygen on recovery from exercise-induced muscle damage in humans. Clin J Sport Med. 2002;12(3):139–50.
Xu HD, Qin Z-H. Beclin 1, Bcl-2 and autophagy. Adv Exp Med Biol. 2019;1206:109–26.
You SY, Park YS, Jeon HJ, Cho DH, Jeon HB, Kim SH, Chang JW, Kim JS, Oh JS. Beclin-1 knockdown shows abscission failure but not autophagy defect during oocyte meiotic maturation. Cell Cycle. 2016;15(12):1611– 9.
Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100(25):15077–82.
Acknowledgements
We thank R.W. for the help in building the experimental ideas, we thank the relevant funds for their support to the experiment, and also thank all the research staff in the laboratory for their contributions to the project.
Funding
This work was supported by the Special Funded Project of the Basic Scientific Research Operation Fee of the Central University (Grant No. 2019PT013, Grant No. 2018GJ015) and the Natural Science Foundation of China (Grant No. 31471133).
Author information
Authors and Affiliations
Contributions
J.L., R.W., and D.W. conceived and designed the whole research; D.W., Z.W., and X.W. summarized and collated biochemical index detection and data; Z.K. and D.W. constructed a centrifugal motion model; J.L., Z.W., and X.W. wrote and commented on the paper.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared no conflict of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Wu, D., Wang, Z. et al. The Role of Autophagy Regulator HMGB1 in Skeletal Muscle Autophagy After Eccentric Exercise. J. of SCI. IN SPORT AND EXERCISE 5, 280–288 (2023). https://doi.org/10.1007/s42978-022-00182-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42978-022-00182-0