Abstract
Neuroimaging studies investigating the association between mental fatigue (henceforth fatigue) and brain physiology have identified many brain regions that may underly the cognitive changes induced by fatigue. These studies focused on the functional changes and functional connectivity of the brain relating to fatigue. The structural correlates of fatigue, however, have received little attention. To fill this gap, this study explored the associations of fatigue with cortical thickness of frontal and parietal regions. In addition, we aimed to explore the associations between reward-induced improvement in performance and neuroanatomical markers in fatigued individuals. Thirty-nine healthy volunteers performed the psychomotor vigilance task for 15 min (i.e., 3 time-on-task blocks of 5 min) out of scanner; followed by an additional rewarded block of the task lasting 5 min. Baseline high-resolution T1-weigthed MR images were obtained. Reaction time increased with time-on-task but got faster again in the rewarded block. Participants’ subjective fatigue increased during task performance. In addition, we found that higher increase in subjective mental fatigue was associated with the cortical thickness of the following areas: bilateral precuneus, right precentral gyrus; right pars triangularis and left superior frontal gyrus. Our results suggest that individual differences in subjective mental fatigue may be explained by differences in the degree of cortical thickness of areas that are associated with motor processes, executive functions, intrinsic alertness and are parts of the default mode network.
Similar content being viewed by others
Introduction
Mental fatigue (henceforward: fatigue) is a very common phenomenon that refers to a psychobiological state that results from prolonged mental activity and is usually associated with feelings of exhaustion, tiredness, reduced motivation, and impaired cognitive functioning (Hockey 2011; Lorist et al. 2005; van der Linden 2011). Deteriorating effects of fatigue are often enhanced with increasing time spent on a cognitive task (see e.g., Matuz et al. 2019, 2022a; Möckel et al. 2015). This phenomenon is usually denoted in the literature as the Time-on-Task (ToT) effect and can be manifested in both subjective and objective markers (e.g., increased response time, altered brain activity) (Ackerman et al. 2010; Darnai et al. 2023; Hopstaken et al. 2015a). The fatigue resulted from ToT is often described as a stop-signal (van der Linden 2011) that warns the individual when the expected energetical costs of task performance outweigh its expected benefits (Boksem and Tops 2008). In line with this, fatigue has been associated with the feeling of reduced motivation and willingness to continue the cognitive task at hand (Hopstaken et al. 2015a, b).
It is well known that fatigue induced by ToT is associated with not only psychological but significant biological changes as well (Boksem et al. 2006; Ishii et al. 2014; Lorist et al. 2005). More specifically, to mention a few, fatigue has been associated with decreased pupil size (Hopstaken et al. 2015b), increased eye blinking frequency (Martins and Carvalho 2015), increased heart rate variability (Matuz et al. 2021) and altered brain activity (Tran et al. 2020). As the aim of the present study was the investigation of structural neural correlates of fatigue, the most relevant previous findings are that showed associations between fatigue and brain activity measured by MRI. Functional MRI studies reported that the activation of various cortical and sub-cortical areas (e.g., middle frontal, pre/postcentral gyri, insular cortex) decreased with ToT (Asplund and Chee 2013; Darnai et al. 2023; Lim et al. 2010, but see Gui et al. 2015). Most recently, for example, Darnai et al. (2023) found that the activity of many frontal and parietal areas (e.g., inferior and superior frontal gyri, bilateral supramarginal gyrus) that are responsible for attention, cognitive control, motor processes, spatial processing (i.e., processes involved in task performance) decreased as participants spent more time on the fatiguing psychomotor vigilance task (PVT). In addition, it has also been shown that the activation of areas such as the middle frontal gyrus (MFG), insula and anterior cingular cortex that are involved in motivational processes also decreased over time (Darnai et al. 2023).
When investigating individual differences in fatigue, Lim et al. (2010) found that resting cerebral blood flow in the middle frontal gyrus was positively associated with the increase in reaction time during the PVT task. A relationship between fatigue and resting activity of the default mode network (DMN) has also been found (Gergelyfi et al. 2021; Gui et al. 2015). DMN is a brain network known to be activated during rest but deactivated during task performance (Raichle 2015). Higher activity of the DMN was associated with higher subjective fatigue (Gergelyfi et al. 2021) and larger ToT-related slowing in reaction times (Gui et al. 2015). To conclude, empirical evidence supports the notion that individual differences in fatigue are associated with functional neural processes. However, it is an interesting and yet unanswered question whether individual differences in ToT-induced fatigue have structural neural correlates (e.g., the correlation with cortical thickness, a measure of columnar organization of the cortex). In fact, this question is highly relevant for research and practice alike as identifying the structural correlates of fatigue not only would help us deepen our understanding of the phenomenon but also would make the prediction of fatigue sensitivity more effective. To date, only a few studies have investigated the structural neural correlates of fatigue resulted from prolonged cognitive task performance. Recently, Román et al. (2022) tested the relationship between white matter microstructure and fatigue induced by a prolonged switching task in patients with multiple sclerosis. They found that lower white matter integrity in several white matter tracts was positively associated with a steeper increase in subjective fatigue. Another study showed that the decline in the performance of a prolonged vigilance task was associated with total brain white matter lesion volumes and grey matter lesions in the right frontal lobe in patients with traumatic brain injury (Schönberger et al. 2017). It is important to point out that these studies included clinical samples only and thus, these results cannot be generalized to the healthy population. To our best knowledge, no previous study explored the structural neural correlates of fatigue induced by ToT in healthy individuals. A potential reason for the gap in literature might be the general lack of between-subject studies in the fatigue literature as most studies primarily focused on the within-subject changes related to fatigue (see also Discussion).
As mentioned earlier, brain areas related to motivation has been also shown to be associated with fatigue. The pivotal role of motivational processes in fatigue has been supported by several lines of empirical evidence (Boksem et al. 2006; Darnai et al. 2023; Hopstaken et al. 2015a, b; Lorist et al. 2009, but see Gergelyfi et al. 2021). Hopstaken et al. (2015a, b), for example, showed that introducing a time reward (i.e., the promise of early finish) restored the detrimental effects of fatigue even after two hours of cognitive performance. Similar results were found when 15-min of continuous performance of the PVT task was followed by the introduction of monetary reward (Darnai et al. 2023). These results support the notion that the emergence of fatigue is associated with an imbalance between the expected costs and benefits of task performance (Boksem and Tops 2008). As providing an extra reward resulted in restored task performance, it can be assumed that the expected costs and benefits had become more balanced (i.e., due to the increase in the expected benefits, while the expected costs remained the same). On the neural level, the restoration of cognitive performance has been linked to several brain areas including the MFG (Darnai et al. 2023), an area that might play an important role in fatigue-related motivational processes (Müller and Apps 2019).
To summarize, this study aimed to fill in the gap in literature by exploring the relationship between cortical thickness and fatigue induced by a prolonged vigilance task, the PVT task, in healthy individuals. The PVT is a widely used task (e.g., Dimitrakopoulos et al. 2018; Möller et al. 2017; Smith et al. 2019) in fatigue studies. The frequent use of PVT is possibly due to its high sensitivity to fatigue (Lim et al. 2012; Sun et al. 2014). It has also been argued that PVT is cognitively very demanding and requires strong cognitive control (Langner and Eickhoff 2013; Lim et al. 2012). The main goal was to identify further biological factors that might explain the individual differences in task-induced fatigue. In addition, we also explored the structural neural correlates of reward-induced changes in task performance. To achieve this, after the fatigue induction, a time reward was introduced to the participants to increase the level of motivation and we investigated the association between the magnitude of change in task performance and the cortical thickness of frontal and parietal brain areas. We decided to focus on these areas for two reasons. First, multiple lines of empirical evidence support the notion that there is strong relationship between ToT-induced fatigue and frontal and parietal areas (e.g., Lim et al. 2010; Sun et al. 2014; Taya et al. 2018; Trejo et al. 2015). Second, recently, Darnai et al. showed that these areas are related to not only ToT-induced fatigue but also reward-related changes, which are also in the focus of the current study. For example, while the activation of the MFG decreased with ToT, after a motivational manipulation, its activation increased (Darnai et al. 2023).
Materials and methods
Participants
As part of a larger protocol, forty-four volunteers were involved in the study. The data of five participants had to be dropped due to technical failures or problems with MRI images. Thus, the final dataset consisted of thirty-nine participants (24 female, 1 left-handed, aged between 18 and 30 years, M = 22.68, SD = 3.2). All of them had normal or corrected-to-normal vision and none of them reported any neurological or psychiatric history. Participants were screened for neurological and psychiatric problems via telephone interview prior to the experiment. They provided informed consent to the protocol that has been approved by the National Medical Research Council (registration number: 6843- 5/2021/EÜIG).
Sample size justification
The sample size was determined based on a priori power analysis conducted by using Gpower 3.1 (Faul et al. 2007). In general, our aim was to achieve a power of 90% with alpha set to 5%. Sample size calculation for the ToT effect in the PVT task was based on previous studies that used the same task and had similar aims. These studies reported large effects sizes (ηp2 = 0.29–0.57; Darnai et al. 2023; Steinborn et al. 2016) and by applying these, the minimum required sample size was 5. For the reward-effect, the estimation was based on studies using the PVT task or investigating the effects of reward in fatigued participants (Hopstaken et al. 2015a, 2015b; Massar et al. 2018). Applying the effect sizes reported (ηp2 = 0.20–0.60), the minimum required sample size was also 5. Finally, for the sample size calculation of the structural neural correlates of fatigue and reward, the power analysis was based on the studies of Lim et al., (2010) and Gergelyfi et al. (2021). In these studies, r-values of significant associations ranged between 0.44 and 0.70. The correlates of fatigue in our study were explored by a series of linear regression analyses conducted separately on each region of interest (see also Data analysis). By applying the r-values reported by the abovementioned studies (with the number of predictors = 4, that is, age, sex, sleep duration and cortical thickness of one area), the required minimum sample size was 32 to detect the associations between cortical thickness and fatigue and reward related effects. To conclude, the final sample of 39 participants had the appropriate statistical power to detect the effects we aimed to investigate.
Task and procedure
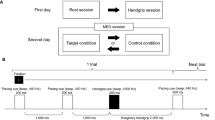
The PVT was administered out-of-scanner using PsychoPy 3 (version 3.0.6. for Windows, Peirce 2007, 2009) for stimulus presentation. Each trial started with a fixation cross (occupying 1.2° of the visual angle) presented at the centre of the monitor. The target stimulus was a running millisecond counter that appeared after a random interval of 2–10 s drawn from a uniform distribution. Participants were instructed to stop the millisecond counter by pressing the ‘Space’ button as quickly as possible. Upon button press, the millisecond counter remained on the screen for 1 s providing visual feedback on reaction time. The next trial started after the feedback disappeared. Figure 1 schematizes the sequence of a trial.
Participants were asked to have an adequate sleep during the night prior to the experiment. Upon arrival to the laboratory, participants were given a consent form to read and sign and their sleep duration was assessed by self-report (the mean sleep duration was 7.48 h with an SD of 1.57). After that, the task was explained to the participants, and they also had a few practice trials. Prior to the PVT task, they were asked to indicate their subjective level of fatigue on a 100 mm visual analogue scale (VASbefore). The scale was presented on the screen with the labels “No fatigue at all” on left side and “Very severe fatigue” on the right side. Participants performed 3 blocks of 44 trials of the PVT. This whole period lasted approx. 15 min.
Immediately after the third block of trials (i.e., at the end of the fatigue induction), participants’ subjective experience with fatigue was registered for the second time on the same scale (VASafter), and then the time-reward manipulation was introduced. We used a similar protocol as described in Hopstaken et al. (2015a, b) combined with the RT-based method of rewarding reported in Massar et al. (2018). Participants were given a message on the screen that the remaining time of the experiment would depend on their performance: for each response below a specified reaction time criterion, the remaining time of the experiment decreases by 1 min. Individual criterion was determined as the participant’s median RT over the first three blocks. They were also informed that the remaining time could be as long as 25 min but in reality, the length of the reward block was identical to the preceding blocks (i.e., approx. 5 min). All the other task parameters of the reward block were identical to that of the first three blocks. The motivational instruction remained for 20 s on the screen and then, the fourth block started immediately.
MRI examination
MRI data were collected on a 3 Tesla MR scanner (MAGNETOM Trio a Tim System, Siemens AG, Erlangen, Germany) equipped with a 12-channel head coil. For the morphometric analyses, isotopic 3D T1-weighted magnetization-prepared rapid gradient echo images were used: repetition time/inversion time/echo time = 2530/1100/3.37 ms, slice thickness = 1 mm, number of sagittal slices = 176, flip angle = 7°, receiver bandwidth = 200 Hz/pixel, field of view = 256 × 256 mm2, 256 × 256 matrix.
Freesurfer v6.0 was used for the cortical reconstruction and volumetric segmentation of the images (http://surfer.nmr.mgh.harvard.edu/). Freesurfer is a freely available and widely-used software for MRI data processing (e.g., Dale et al. 1999; Fischl et al. 1999). Freesurfer’s semi-automatic anatomical processing scripts (autorecon 1, 2, and 3) were executed on all the subjects’ data. For all the subjects, the data was visually inspected and manual adjustments were applied when needed. Following Pellicano et al. (2010), we only focused on the frontal and parietal cortical areas. Thus, we defined 15 regions of interest (ROIs) using the Desikan–Killiany–Tourville atlas (Klein and Tourville 2012) implemented in Freesurfer. The Desikan-Kiliany-Tourville protocol is a modified version of the Desikan-Killiany labelling protocol, which relies on region boundaries that are approximated by surface depth and curvature, and it includes more consistent and unambiguous regions. The improvement of the labelling procedure resulted in a more reliable estimation, thus a higher overlap between automatically and manually labelled cortical regions (Klein and Tourville 2012).The following bilateral subregions of the frontal and parietal lobes were selected for the analyses: caudal and rostal middle frontal gyri, lateral and medial orbitofrontal gyri, precentral and paracentral gyri, pars opercularis, pars orbitalis, pars triangularis, superior frontal gyrus, inferior and superior parietal cortex, postcentral gyrus, precuneus and supramarginal gyrus.
Data analysis
Data preprocessing as well as statistical analyses were carried out in SPSS (version 28, IBM) and in Python using the “statsmodels” package (version 0.13.5., Seabold & Perktold 2010). Subjective fatigue induced by the PVT task was tested by paired samples t-test (VASbefore vs. VASafter). For the analysis of behavioural performance, mean RT was computed for each block of trials. RTs below 100 ms (i.e., anticipatory responses) were excluded from the analyses. There were three such trials in total. There were no omission errors and thus error rates were not analyzed. The ToT-effect on RT was tested by repeated measures ANOVA (rANOVA) with Block (3 levels: the first three blocks of the PVT task) as within-subject factor. Pairwise comparisons were adjusted for multiple comparisons using Bonferroni correction. The reward-effect on RT was tested by paired-samples t-test (Block 3. vs. Block 4.).
To explore the association between cortical thickness and fatigue-, and reward-related changes, a series of multiple linear regression analyses was performed with three outcome variables: change in subjective fatigue, change in RT in the first three blocks, and reward-effect. The change in subjective fatigue was defined as the difference between the two administrations of VAS (VASafter—VASbefore). For the analysis, this difference score was used as an index of ToT—induced fatigue. The change in RT was calculated as the RT difference between the first and third block of the PVT task (Block 3–Block 1), while the reward-effect was calculated as the RT difference between the third block and the reward block (Block 3–Block 4). Multiple linear regression analyses were conducted for each outcome variable and ROI, separately. Sex and age of the participants as well as self-reported sleep duration were added as covariates. We included sleep duration, because it is widely recognized that sleep and fatigue are strongly related and thus, sleep duration might be a confounding variable (e.g., Åkerstedt et al. 2004; Asplund and Chee 2013). The normality assumption of residuals was tested by both visual and statistical tests. Homoscedasticity was tested by the Breusch-Pagan test. If these assumptions were violated, robust regression was conducted instead of ordinary least squares regression (Field and Wilcox 2017). To detect outliers, Mahalanobis distance and Cook’s distance were applied. To adjust for multiple testing, the Benjamini–Hochberg procedure (Benjamini and Hochberg 1995) was applied with the false discovery rate (FDR) set at 5%. Results were considered significant at p < 0.05 after FDR-correction.
Results
Fatigue and reward effects
The analysis of subjective fatigue ratings revealed significantly higher fatigue after the third block of trials (VASafter, M = 47.10, SD = 25.16) compared to fatigue reported prior to the task (VASbefore, M = 28.26, SD = 21.19) suggesting elevated levels of mental fatigue due to the PVT-task (t(38) = 5.40, p < 0.001, Cohen’s d = 0.87). Table 1 and Fig. 2 depicts the results of mean RT over the four blocks of trials. The analysis showed that RT significantly changed over the first three blocks of the PVT task (F(1.71, 64.79) = 16.11, ηp2 = 0.30). Bonferroni-corrected post-hoc analyses showed that mean RT was significantly higher in the third block compared to the first and second blocks (Block 1 vs. Block 2: p = 0.10; Block 1 vs. Block 3: p < 0.001; Block 2 vs. Block 3: p < 0.001). After the reward manipulation, in the fourth block, RT was significantly lower compared to the third block (t(38) = 6.57, p < 0.001, Cohen’s d = 1.05). To conclude, both the fatigue and reward manipulations were successful as indicated by increasing RTs and subjective fatigue during the first three blocks, and decreased RT in the fourth, rewarded block, respectively.
Associations between behavioural measures, subjective fatigue, and cortical thickness
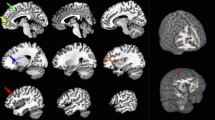
Results of linear regression analyses for each frontal and parietal ROI are summarized in Tables 2 and 3, respectively. Here we report the most important findings. The change in subjective fatigue was negatively associated with the cortical thickness of the bilateral precuneus, right precentral gyrus, right pars triangularis and left superior frontal gyrus (SFG). That is, thinner cortical thickness in these areas were associated with higher levels of fatigue induced by the PVT-task. The RT-based outcome measures, the change in RT over the first three blocks, and the reward-effect, showed no significant association with cortical thickness of our ROIs after correcting for multiple testing. Using a little more liberal threshold (FDR = 10%), however, the reward-effect showed significantly negative associations with the cortical thickness of the left rostral MFG and the left SFG. That is, higher RT improvement in the fourth block was associated with thinner cortical thickness in the two areas.
Discussion
It is an everyday phenomenon that the levels of alertness and attention fluctuate (Ádám, 2004) and it is well known that these fluctuations tend to be enhanced with increasing ToT (see e.g., Lorist et al. 2005; Matuz et al. 2022a). Although the psychological and biological correlates of fatigue resulted from the ToT effect have been extensively investigated, most of these studies applied a within-subject design and relatively little is known about the correlates of interindividual differences in fatigue, which could be tested by applying a cross-sectional design. As a matter of fact, in general, only a few factors have been identified so far that might explain the individual differences in fatigue in the healthy population. The factors that might be potential predictors of fatigue caused by prolonged cognitive activity include sex (Hopko et al. 2021; Matuz et al. 2022b), age (de Jong et al. 2018), dopaminergic polymorphism (Lim et al. 2012), personality traits (Ackerman and Kanfer 2009; Ackerman et al. 2010), oculometrics (Bafna et al. 2021), resting cardiac activity (e.g., Matuz et al. 2022b; Mun and Geng 2019) and resting brain activity (Gergelyfi et al. 2021; Gui et al. 2015; Lim et al. 2010). In a machine learning study, for example, Matuz et al. (2022b) showed that female participants were more likely to indicate higher subjective fatigue induced by a cognitive task. In addition, they also showed that higher resting heart rate variability was associated with higher subjective fatigue (Matuz et al. 2022b). Regarding personality traits, high-anxiety or neuroticism has been found to be positively associated with subjective fatigue (Ackerman and Kanfer 2009; Ackerman et al. 2010).
To extend the literature relating individual differences in fatigue, in this study, we used brain volumetry to measure the cortical thickness of ten frontal and five parietal bilateral areas and investigated whether their cortical thickness might explain individual differences in ToT-induced fatigue. We found negative associations between the increment of subjective mental fatigue and the cortical thickness of brain regions such as the bilateral precuneus, right precentral gyrus, right pars triangularis, and left superior frontal gyrus. In general, these findings are consistent with fMRI studies that showed decreasing activation in these areas as a function of time spent on fatiguing tasks (Asplund and Chee 2013; Darnai et al. 2023; Lim et al. 2010).
Higher increase in ToT-induced subjective fatigue was associated with thinner cortical thickness of the precuneus in both hemispheres. The precuneus is considered a core region of the DMN (Raichle et al. 2001). As mentioned earlier, the connectivity within the DMN is predictive of fatigue (Gergelyfi et al. 2021; Gui et al. 2015). Thus, on the one hand, our findings provide further support for the conclusion that the DMN plays a key role in fatigue. On the other hand, a novel finding of the present study is that the association between the DMN and fatigue was shown on the structural level of the brain in healthy individuals. In clinical studies, the fatigue and precuneus association have already been observed. These studies found decreased cortical thickness in the right precuneus in patients with chronic fatigue symptom (Thapaliya et al. 2022) and multiple sclerosis (MS) (Hanken et al. 2016). MS is a neurodegenerative disease often characterized by increased fatigue (see Oliva Ramirez et al. 2021 for review) and a higher sensitivity to task-induced fatigue indicated by a steeper increase in subjective fatigue over time spent on a cognitive task compared to healthy controls (Sandry et al. 2014). Hence, the negative associations between the cortical thickness of the bilateral precuneus and subjective fatigue shown in the present study are in line with previous clinical studies and suggest that the thinning of the precuneus is likely to be an important factor in fatigue sensitivity.
The cortical thickness of the left superior frontal gyrus (lSFG) also showed a negative association with subjective fatigue in our study. Functional MRI studies showed that the lSFG is involved in processes related to working memory (Courtney et al. 1998), executive functioning (Cutini et al. 2008), and spatial orientation (Boisgueheneuc et al. 2006). Similar to the precuneus, morphometric studies also shed light on the association between grey matter atrophy in lSFG and fatigue in MS patients (Calabrese et al. 2010; Sepulcre et al. 2009). For example, Sepulcre et al. (2009) found that higher levels of self-reported fatigue were associated with atrophy in the lSFG. In addition, they also showed that the degree of atrophy in the lSFG was significantly higher in MS patients with fatigue compared to MS patient without fatigue and healthy controls.
In the present study, two additional frontal structures in the right hemisphere were found to be associated with task-induced increase in subjective fatigue. The precentral gyrus or primary motor cortex is known as one of the areas contributing to voluntary movement control (Stippich et al. 2007). This area has also been linked to both mental and physical fatigue (Darnai et al. 2023; Ishii et al. 2014; Kato et al. 2009; Tanaka et al. 2012). Physical fatigue is usually distinguished from mental fatigue because it is evoked by physical instead of mental work (Hockey 2012). However, it has also been hypothesized that the neural systems associated with physical and mental fatigue may partly overlap (Ishii et al. 2014; Noakes 2008). Ishii et al. (2014), for example, postulated that a dual regulation system involving multiple brain areas (e.g., anterior cingular cortex, insula, basal ganglia) plays a key role in the emergence of both physical and mental fatigue. This system can also affect the function of the primary motor cortex, allowing, among others, motor functions to compensate for the adverse effects of increasing fatigue.
Finally, we found a negative association between subjective fatigue and the cortical thickness of the right pars triangularis. Langner et al. (2012) showed that the right pars triangularis is part of a mainly right-lateralized brain network associated with intrinsic alertness. Intrinsic alertness refers to the capacity of maintaining attention during non-rewarding, monotonous tasks that highly depends on voluntary control of response readiness (Sturm et al. 1999; Sturm and Willmes 2001). Intrinsic alertness has been associated with subjective fatigue: tasks that relied on intrinsic alertness were very likely to induce subjective fatigue (Hanken et al. 2014). For example, increased subjective fatigue was found after the completion of a prolonged pointing task that was demanding in terms of intrinsic alertness (Matuz et al. 2022a). To summarize, our result regarding subjective fatigue and the pars triangularis is consistent with the literature and provides further evidence for the suggested relationship between intrinsic alertness and fatigue.
In sum, the relationship between fatigue and cortical thickness of bilateral precuneus, right precentral and paracentral gyri and the right pars triangularis suggests that the susceptibility to mental fatigue may rely on structural biological substrates that are also influenced by genetical factors (Panizzon et al. 2009). On the one hand, this is an important finding because it provides further support for the notion that fatigue is also affected by trait-like factors and that individual differences in fatigue can be explained by structural biological characteristics. On the other hand, it has several implications for both the clinical and healthy populations as the cortical thickness of frontal and parietal areas are often altered by normal aging (Salat et al. 2004; Thambisetty et al. 2010) and several pathologies like Parkinson’s disease (Jubault et al. 2011) and amyotrophic lateral sclerosis (Consonni et al. 2020).
While the present findings provide additional information on the relationship between fatigue and certain brain regions, we can further speculate on the more general question of how the subjective experience of fatigue is associated with cortical thickness. A potential explanation for the negative relationship between subjective fatigue and cortical thickness could be that higher cortical thickness might indicate higher capacity to compensate fatigue as it reflects more synapses per neuron (Anderson et al. 2002). According to Hockey’s theory, the subjective experience of fatigue emerges when a conflict arises between competing goals (e.g. performing the task well vs. other goals, for example, resting or mind-wandering) but importantly, the conflict can be resolved by compensatory mechanisms (Hockey 1997; 2011). Thus, one might argue that a higher capacity for compensatory mechanisms indicated by better connectivity predicts relatively lower levels of task-induced subjective fatigue. It is important to note, however, that our research design and data analysis do not allow us to draw any far-reaching conclusions about this relationship and further studies are needed to understand how structural brain features relate to fatigue.We used a timed-based reward manipulation in order to test the effects of reward on task performance and to investigate its structural neural correlates. Compared to the third block of trials, participants responded faster in the fourth, rewarded block. Thus, similarly to Hopstaken et al. (2015a, b), our time-based reward manipulation restored task performance. This result is also in line with other studies using the PVT task and monetary reward as a motivational manipulation (Darnai et al. 2023; Massar et al. 2018). It is therefore suggested that the different types of reward (i.e., monetary and time rewards) may be similarly effective in restoring the cognitive performance.
The structural neural correlates of the reward-effect were also investigated and when applying a more liberal threshold, we found a negative relationship between the cortical thickness of the lSFG, left rostral MFG and the reward-induced improvement in task performance. That is, thinner cortical gray matter in these areas predicted higher improvement in the rewarded block. According to fMRI studies, these areas are responsible for attentional processes and executive functions (Cutini et al. 2008; Olesen et al. 2004) and it has also been shown that activation in the MFG increases when reward is introduced to the participants after a fatiguing task (Darnai et al. 2023). Based on these previous fMRI findings, a positive instead of negative relationship between cortical thickness and the reward-effect would probably be more plausible. A potential explanation for our results is that individuals with thinner cortical thickness in the lSFG and left rostral MFG also show higher levels of reward sensitivity (Adrián-Ventura et al. 2019), a trait that might be a key factor in reward-related improvement in performance. Thus, it is possible that individuals with thinner cortical thickness in the lSFG and left rostral MFG might be more sensitive to rewards and therefore, they showed greater improvement in the rewarded block. We need to note, however, that these results were obtained when applying a more liberal FDR (i.e., an FDR of 10% instead of 5%).
Limitations
Although the present study adds further insight into the relationship between neuroanatomy and fatigue, this study had several limitations as well that need to be admitted. First, although several factors were taken into account in the sample size calculation and very conservative FDR was applied, the sample size calculation did not include the number of tests as a factor and therefore, there is still a risk of underpowered statistical analysis. Second, we cannot completely rule out the possibility that the observed ToT effects were at least partially due to other factors (e.g. boredom or time awake) than fatigue. We must point out, however, that it is less likely that other factors significantly affected the subjective fatigue ratings too. Finally, a third limitation of the study is that subjective fatigue was not measured after the rewarded block, however, it is possible that the reward had an effect on the subjective aspect of fatigue as well. Future studies might consider investigating this effect too.
Conclusions for future biology
In summary, we found that individual differences in subjective mental fatigue induced by prolonged cognitive performance are associated with neuroanatomical differences in the frontal and parietal cortex. More specifically, subjective fatigue was negatively related to the cortical thickness of the bilateral precuneus, right precentral and paracentral gyri and the right pars triangularis. These findings provide new perspectives for the future of research and practice alike. For example, the investigation of the effects of cognitive training on the susceptibility to fatigue is an interesting question future studies might aim to answer. Based on the plasticity of grey matter, it could be hypothesized that cognitive training methods that enhance the thickening of the areas associated with fatigue might decrease the susceptibility to fatigue. Nevertheless, our findings are worth for broader follow-up. Future studies might consider using, for example, different cognitive tasks relying on different cognitive operations and explore the structural neural correlates of fatigue induced by them.
References
Ackerman P, Kanfer R (2009) Test length and cognitive fatigue: an empirical examination of effects on performance and test-taker reactions. J Exp Psychol Appl 15:163–181. https://doi.org/10.1037/a0015719
Ackerman PL, Kanfer R, Shapiro SW, Newton S, Beier ME (2010) Cognitive fatigue during testing: an examination of trait, time-on-task, and strategy influences. Hum Perform 23(5):381–402. https://doi.org/10.1080/08959285.2010.517720
Ádám, G. (2004). A rejtőzködő elme. Vince Kiadó.
Adrián-Ventura J, Costumero V, Parcet MA, Ávila C (2019) Linking personality and brain anatomy: a structural MRI approach to reinforcement sensitivity theory. Soc Cognit Affect Neurosci 14(3):329–338. https://doi.org/10.1093/scan/nsz011
Åkerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G (2004) Mental fatigue, work and sleep. J Psychosom Res 57(5):427–433. https://doi.org/10.1016/j.jpsychores.2003.12.001
Anderson BJ, Eckburg PB, Relucio KI (2002) Alterations in the thickness of motor cortical subregions after motor-skill learning and exercise. Learn Mem 9(1):1–9. https://doi.org/10.1101/lm.43402
Asplund CL, Chee MWL (2013) Time-on-task and sleep deprivation effects are evidenced in overlapping brain areas. Neuroimage 82:326–335. https://doi.org/10.1016/j.neuroimage.2013.05.119
Bafna T, Bækgaard P, Hansen JP (2021) Mental fatigue prediction during eye-typing. PLoS ONE 16(2):e0246739. https://doi.org/10.1371/journal.pone.0246739
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statist Soc Ser B (Methodol) 57(1):289–300
Boksem MAS, Tops M (2008) Mental fatigue: costs and benefits. Brain Res Rev 59(1):125–139. https://doi.org/10.1016/j.brainresrev.2008.07.001
Boksem MAS, Meijman TF, Lorist MM (2006) Mental fatigue, motivation and action monitoring. Biol Psychol 72(2):123–132. https://doi.org/10.1016/j.biopsycho.2005.08.007
Calabrese M, Rinaldi F, Grossi P, Mattisi I, Bernardi V, Favaretto A, Perini P, Gallo P (2010) Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing—remitting multiple sclerosis. Mult Scler J 16(10):1220–1228. https://doi.org/10.1177/1352458510376405
Consonni M, Dalla Bella E, Contarino VE, Bersano E, Lauria G (2020) Cortical thinning trajectories across disease stages and cognitive impairment in amyotrophic lateral sclerosis. Cortex 131:284–294. https://doi.org/10.1016/j.cortex.2020.07.007
Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV (1998) An area specialized for spatial working memory in human frontal cortex. Science 279(5355):1347–1351. https://doi.org/10.1126/science.279.5355.1347
Cutini S, Scatturin P, Menon E, Bisiacchi PS, Gamberini L, Zorzi M, Dell’Acqua R (2008) Selective activation of the superior frontal gyrus in task-switching: an event-related fNIRS study. Neuroimage 42(2):945–955. https://doi.org/10.1016/j.neuroimage.2008.05.013
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage 9(2):179–194. https://doi.org/10.1006/nimg.1998.0395
Darnai G, Matuz A, Alhour HA, Perlaki G, Orsi G, Arató Á, Szente A, Áfra E, Nagy SA, Janszky J, Csathó Á (2023) The neural correlates of mental fatigue and reward processing: a task-based fMRI study. Neuroimage 265:119812. https://doi.org/10.1016/j.neuroimage.2022.119812
de Jong M, Jolij J, Pimenta A, Lorist MM (2018) Age modulates the effects of mental fatigue on typewriting. Front Psychol. https://doi.org/10.3389/fpsyg.2018.01113
Dimitrakopoulos GN, Kakkos I, Dai Z, Wang H, Sgarbas K, Thakor N, Bezerianos A, Sun Y (2018) Functional connectivity analysis of mental fatigue reveals different network topological alterations between driving and vigilance tasks. IEEE Trans Neural Syst Rehabil Eng 26(4):740–749. https://doi.org/10.1109/TNSRE.2018.2791936
du Boisgueheneuc F, Levy R, Volle E, Seassau M, Duffau H, Kinkingnehun S, Samson Y, Zhang S, Dubois B (2006) Functions of the left superior frontal gyrus in humans: a lesion study. Brain 129(12):3315–3328. https://doi.org/10.1093/brain/awl244
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191. https://doi.org/10.3758/BF03193146
Field AP, Wilcox RR (2017) Robust statistical methods: a primer for clinical psychology and experimental psychopathology researchers. Behav Res Ther 98:19–38. https://doi.org/10.1016/j.brat.2017.05.013
Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis: ii: inflation, flattening, and a surface-based coordinate system. Neuroimage 9(2):195–207. https://doi.org/10.1006/nimg.1998.0396
Gergelyfi M, Sanz-Arigita EJ, Solopchuk O, Dricot L, Jacob B, Zénon A (2021) Mental fatigue correlates with depression of task-related network and augmented DMN activity but spares the reward circuit. Neuroimage 243:118532. https://doi.org/10.1016/j.neuroimage.2021.118532
Gui D, Xu S, Zhu S, Fang Z, Spaeth AM, Xin Y, Feng T, Rao H (2015) Resting spontaneous activity in the default mode network predicts performance decline during prolonged attention workload. Neuroimage 120:323–330. https://doi.org/10.1016/j.neuroimage.2015.07.030
Hanken K, Eling P, Hildebrandt H (2014) The representation of inflammatory signals in the brain–a model for subjective fatigue in multiple sclerosis. Front Neurol 5:264. https://doi.org/10.3389/fneur.2014.00264
Hanken K, Eling P, Klein J, Klaene E, Hildebrandt H (2016) Different cortical underpinnings for fatigue and depression in MS? Multiple Scler Relat Disord 6:81–86. https://doi.org/10.1016/j.msard.2016.02.005
Hockey G (1997) Compensatory control in the regulation of human performance under stress and high workload: a cognitive-energetical framework. Biol Psychol 45(1):73–93. https://doi.org/10.1016/S0301-0511(96)05223-4
Hockey B (2012) Challenges in fatigue and performance research. The handbook of operator fatigue. CRC Press, Boca Raton, pp 45–60
Hockey GRJ (2011) A motivational control theory of cognitive fatigue. In PL Ackerman (Ed.), Cognitive fatigue: multidisciplinary perspectives on current research and future applications. (pp. 167–187). American Psychological Association. https://doi.org/10.1037/12343-008
Hopko SK, Khurana R, Mehta R, Pagilla P (2021) Effect of cognitive fatigue, operator sex, and robot assistance on task performance metrics, workload, and situation awareness in human-robot collaboration. IEEE Robot Autom Lett 6:3049–3056. https://doi.org/10.1109/LRA.2021.3062787
Hopstaken JF, van der Linden D, Bakker AB, Kompier MAJ (2015a) A multifaceted investigation of the link between mental fatigue and task disengagement. Psychophysiology 52(3):305–315. https://doi.org/10.1111/psyp.12339
Hopstaken JF, van der Linden D, Bakker AB, Kompier MAJ (2015b) The window of my eyes: task disengagement and mental fatigue covary with pupil dynamics. Biol Psychol 110:100–106. https://doi.org/10.1016/j.biopsycho.2015.06.013
Ishii A, Tanaka M, Watanabe Y (2014) Neural mechanisms of mental fatigue. Rev Neurosci 25(4):469–479. https://doi.org/10.1515/revneuro-2014-0028
Jubault T, Gagnon J-F, Karama S, Ptito A, Lafontaine A-L, Evans AC, Monchi O (2011) Patterns of cortical thickness and surface area in early Parkinson’s disease. Neuroimage 55(2):462–467. https://doi.org/10.1016/j.neuroimage.2010.12.043
Kato Y, Endo H, Kizuka T (2009) Mental fatigue and impaired response processes: event-related brain potentials in a Go/NoGo task. Int J Psychophysiol 72(2):204–211. https://doi.org/10.1016/j.ijpsycho.2008.12.008
Klein A, Tourville J (2012) 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci 6:171. https://doi.org/10.3389/fnins.2012.00171
Langner R, Eickhoff SB (2013) Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull 139(4):870–900. https://doi.org/10.1037/a0030694
Langner R, Kellermann T, Eickhoff SB, Boers F, Chatterjee A, Willmes K, Sturm W (2012) Staying responsive to the world: modality-specific and -nonspecific contributions to speeded auditory, tactile, and visual stimulus detection. Hum Brain Mapp 33(2):398–418. https://doi.org/10.1002/hbm.21220
Lim J, Wu W, Wang J, Detre JA, Dinges DF, Rao H (2010) Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage 49(4):3426–3435. https://doi.org/10.1016/j.neuroimage.2009.11.020
Lim J, Ebstein R, Tse C-Y, Monakhov M, Lai PS, Dinges DF, Kwok K (2012) Dopaminergic polymorphisms associated with time-on-task declines and fatigue in the psychomotor vigilance test. PLoS ONE 7(3):e33767. https://doi.org/10.1371/journal.pone.0033767
van der Linden, D. (2011). The urge to stop: The cognitive and biological nature of acute mental fatigue. In Cognitive fatigue: Multidisciplinary perspectives on current research and future applications (pp. 149–164). American Psychological Association. https://doi.org/10.1037/12343-007
Lorist MM, Boksem MAS, Ridderinkhof KR (2005) Impaired cognitive control and reduced cingulate activity during mental fatigue. Cogn Brain Res 24(2):199–205. https://doi.org/10.1016/j.cogbrainres.2005.01.018
Lorist MM, Bezdan E, ten Caat M, Span MM, Roerdink JBTM, Maurits NM (2009) The influence of mental fatigue and motivation on neural network dynamics; an EEG coherence study. Brain Res 1270:95–106. https://doi.org/10.1016/j.brainres.2009.03.015
Martins R, Carvalho J (2015) Eye blinking as an indicator of fatigue and mental load–a systematic review. Occupat Safety Hyg III 10:231. https://doi.org/10.1201/b18042-48
Massar SAA, Sasmita K, Lim J, Chee MWL (2018) Motivation alters implicit temporal attention through sustained and transient mechanisms: a behavioral and pupillometric study. Psychophysiology 55(12):e13275. https://doi.org/10.1111/psyp.13275
Matuz A, Van der Linden D, Topa K, Csathó Á (2019) Cross-modal conflict increases with time-on-task in a temporal discrimination task. Front Psychol. https://doi.org/10.3389/fpsyg.2019.02429
Matuz A, van der Linden D, Kisander Z, Hernádi I, Kázmér K, Csathó Á (2021) Enhanced cardiac vagal tone in mental fatigue: analysis of heart rate variability in time-on-task, recovery, and reactivity. PLoS ONE 16(3):e0238670. https://doi.org/10.1371/journal.pone.0238670
Matuz A, van der Linden D, Darnai G, Csathó Á (2022) Generalisable machine learning models trained on heart rate variability data to predict mental fatigue. Sci Rep 12(1):1014. https://doi.org/10.1038/s41598-022-24415-y
Matuz A, van der Linden D, Zsidó A, Csathó Á (2022b) Visually guided movement with increasing time-on-task: differential effects on movement preparation and movement execution. Quart J Exp Psychol 75(4):565–582. https://doi.org/10.1177/17470218211048001
Möckel T, Beste C, Wascher E (2015) The effects of time on task in response selection—an ERP study of mental fatigue. Sci Rep 5(1):1204. https://doi.org/10.1038/srep10113
Möller MC, Nordin LE, Bartfai A, Julin P, Li T-Q (2017) Fatigue and cognitive fatigability in mild traumatic brain injury are correlated with altered neural activity during vigilance test performance. Front Neurol. https://doi.org/10.3389/fneur.2017.00496
Müller T, Apps MAJ (2019) Motivational fatigue: a neurocognitive framework for the impact of effortful exertion on subsequent motivation. Neuropsychologia 123:141–151. https://doi.org/10.1016/j.neuropsychologia.2018.04.030
Mun E-Y, Geng F (2019) Predicting post-experiment fatigue among healthy young adults: Random forest regression analysis. Psychol Test Assess Model 61(4):471–493
Noakes TD (2008) Testing for maximum oxygen consumption has produced a brainless model of human exercise performance. Br J Sports Med 42(7):551–555. https://doi.org/10.1136/bjsm.2008.046821
Olesen PJ, Westerberg H, Klingberg T (2004) Increased prefrontal and parietal activity after training of working memory. Nat Neurosci 7(1):75–79. https://doi.org/10.1038/nn1165
Oliva Ramirez A, Keenan A, Kalau O, Worthington E, Cohen L, Singh S (2021) Prevalence and burden of multiple sclerosis-related fatigue: a systematic literature review. BMC Neurol 21(1):468. https://doi.org/10.1186/s12883-021-02396-1
Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS (2009) Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 19(11):2728–2735. https://doi.org/10.1093/cercor/bhp026
Peirce JW (2007) PsychoPy—psychophysics software in python. J Neurosci Methods 162(1):8–13. https://doi.org/10.1016/j.jneumeth.2006.11.017
Peirce JW (2009) Generating stimuli for neuroscience using psychopy. Front Neuroinformatics. https://doi.org/10.3389/neuro.11.010.2008
Raichle ME (2015) The brain’s default mode network. Annu Rev Neurosci 38:433–447. https://doi.org/10.1146/annurev-neuro-071013-014030
Román CAF, Wylie GR, DeLuca J, Yao B (2022) Associations of white matter and basal ganglia microstructure to cognitive fatigue rate in multiple sclerosis. Front Neurol. https://doi.org/10.3389/fneur.2022.911012
Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B (2004) Thinning of the cerebral cortex in aging. Cereb Cortex 14(7):721–730. https://doi.org/10.1093/cercor/bhh032
Sandry J, Genova HM, Dobryakova E, DeLuca J, Wylie G (2014) Subjective cognitive fatigue in multiple sclerosis depends on task length. Front Neurol. https://doi.org/10.3389/fneur.2014.00214
Schönberger M, Reutens D, Beare R, O’Sullivan R, Rajaratnam SMW, Ponsford J (2017) Brain lesion correlates of fatigue in individuals with traumatic brain injury. Neuropsychol Rehabil 27(7):1056–1070. https://doi.org/10.1080/09602011.2016.1154875
Seabold S, Perktold J (2010) Statsmodels: econometric and statistical modeling with python. pp 92–96. https://doi.org/10.25080/Majora-92bf1922-011
Sepulcre J, Masdeu J, Goñi J, Arrondo G, Vélez de Mendizábal N, Bejarano B, Villoslada P (2009) Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler J 15(3):337–344. https://doi.org/10.1177/1352458508098373
Smith MR, Chai R, Nguyen HT, Marcora SM, Coutts AJ (2019) Comparing the effects of three cognitive tasks on indicators of mental fatigue. J Psychol 153(8):759–783. https://doi.org/10.1080/00223980.2019.1611530
Steinborn MB, Langner R, Flehmig HC, Huestegge L (2016) Everyday life cognitive instability predicts simple reaction time variability: analysis of reaction time distributions and delta plots. Appl Cogn Psychol 30(1):92–102. https://doi.org/10.1002/acp.3172
Stippich C, Blatow M, Durst A, Dreyhaupt J, Sartor K (2007) Global activation of primary motor cortex during voluntary movements in man. Neuroimage 34(3):1227–1237. https://doi.org/10.1016/j.neuroimage.2006.08.046
Sturm W, Willmes K (2001) On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage 14(1):S76–S84. https://doi.org/10.1006/nimg.2001.0839
Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, Herzog H, Tellmann L, Müller-Gärtner H-W, Willmes K (1999) Functional anatomy of intrinsic alertness: evidencefor a fronto-parietal-thalamic-brainstem network in theright hemisphere. Neuropsychologia 37(7):797–805. https://doi.org/10.1016/S0028-3932(98)00141-9
Sun Y, Lim J, Kwok K, Bezerianos A (2014) Functional cortical connectivity analysis of mental fatigue unmasks hemispheric asymmetry and changes in small-world networks. Brain Cogn 85:220–230. https://doi.org/10.1016/j.bandc.2013.12.011
Tanaka M, Shigihara Y, Ishii A, Funakura M, Kanai E, Watanabe Y (2012) Effect of mental fatigue on the central nervous system: an electroencephalography study. Behav Brain Funct 8(1):48. https://doi.org/10.1186/1744-9081-8-48
Taya F, Dimitriadis SI, Dragomir A, Lim J, Sun Y, Wong KF, Thakor NV, Bezerianos A (2018) Fronto-parietal subnetworks flexibility compensates for cognitive decline due to mental fatigue. Hum Brain Mapp 39(9):3528–3545. https://doi.org/10.1002/hbm.24192
Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM (2010) Longitudinal changes in cortical thickness associated with normal aging. Neuroimage 52(4):1215–1223. https://doi.org/10.1016/j.neuroimage.2010.04.258
Thapaliya K, Marshall-Gradisnik S, Staines D, Su J, Barnden L (2022) Alteration of cortical volume and thickness in myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurosci. https://doi.org/10.3389/fnins.2022.848730
Tran Y, Craig A, Craig R, Chai R, Nguyen H (2020) The influence of mental fatigue on brain activity: evidence from a systematic review with meta-analyses. Psychophysiology 57(5):e13554. https://doi.org/10.1111/psyp.13554
Trejo LJ, Kubitz K, Rosipal R, Kochavi RL, Montgomery LD (2015) EEG-based estimation and classification of mental fatigue. Psychology 06(05):572. https://doi.org/10.4236/psych.2015.65055
Acknowledgements
AM was supported by the ÚNKP-19-3 New National Excellence Program of the Ministry for Innovation and Technology. AM, AZ, GD, and AC were supported by the National Research, Development and Innovation Office, NKFIH grant (K142321).
Funding
Open access funding provided by University of Pécs.
Author information
Authors and Affiliations
Contributions
AM and AC designed and performed the experiments, analysed data and wrote the paper. GD and AZ analysed and interpreted the data. JJ wrote the paper. All authors discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matuz, A., Darnai, G., Zsidó, A.N. et al. Structural neural correlates of mental fatigue and reward-induced improvement in performance. BIOLOGIA FUTURA (2023). https://doi.org/10.1007/s42977-023-00187-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42977-023-00187-y