Article contents
The Composition and Growth Mechanism of Coexisting 4M2 and 4A8 Biotite Polytypes from Rhyolite of Long Valley Caldera, California
Published online by Cambridge University Press: 01 January 2024
Abstract
Polytypism is common in micas, and the frequency of polytype occurrence is believed to be related closely to the crystallization conditions and chemical compositions of the corresponding fluids and melts. Coexisting multiple standard and complex/disordered polytypes in igneous rocks generally reflect a complicated magma evolution history. The purpose of the current study was to clarify the origin of coexisting biotite polytypes and their growth mechanism. Micro-X-ray diffraction (μXRD) and transmission electron microscopy (TEM) were used to investigate Fe-rich biotite phenocrysts in rhyolite from the Long Valley Caldera, California, USA. The μXRD analyses characterized various polytypes, and TEM observations revealed that common polytypes (e.g. 1M, 2M1, and 3T) and rare polytypes (e.g. 4M2 and 4A8) coexist within biotite monocrystals. The two 4-layer polytypes of Fe-rich biotite, 4M2 and 4A8, were identified via selected-area electron diffraction (SAED) and high-resolution scanning transmission electron microscopy (HRSTEM) at the atomic resolution, with unique stacking sequences ([0222] for 4M2 and [0022¯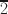 ] for 4A8). Energy-dispersive X-ray spectroscopy (EDS) results showed differences in their chemical compositions, especially Fe and K. The 4A8 polytype is reported for the first time. The present study suggested that environmental changes, such as rapid cooling and inhomogeneous compositional distribution, led to chemical and structural oscillations and complex nucleation of the two 4-layer polytypes. Screw dislocations producing spiral growth enhance polytype stability and form ordered long-period/complex polytypes. These results are useful to understand the origin of long-period/complex polytypes and the intergrowths of diverse polytypes formed in non-equilibrium crystallization environments.
] for 4A8). Energy-dispersive X-ray spectroscopy (EDS) results showed differences in their chemical compositions, especially Fe and K. The 4A8 polytype is reported for the first time. The present study suggested that environmental changes, such as rapid cooling and inhomogeneous compositional distribution, led to chemical and structural oscillations and complex nucleation of the two 4-layer polytypes. Screw dislocations producing spiral growth enhance polytype stability and form ordered long-period/complex polytypes. These results are useful to understand the origin of long-period/complex polytypes and the intergrowths of diverse polytypes formed in non-equilibrium crystallization environments.
- Type
- Original Paper
- Information
- Copyright
- Copyright © The Clay Minerals Society 2022
References
- 2
- Cited by




