Abstract
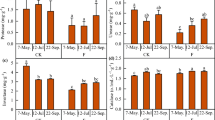
Sulfentrazone (STZ) is an efficient tool for the pre- and post-emergence control of monocotyledonous and dicotyledonous weeds in fields of crops such as pineapple, coffee, sugarcane, citrus, eucalyptus, tobacco, and soybean. However, this herbicide persists in the soil, causing phytotoxicity in the subsequent crop. Therefore, it is important to use efficient strategies for the remediation of STZ-contaminated areas. The aim of this study was to evaluate the effects of Crotalaria juncea L. on the remediation of STZ-contaminated soil and on the microbial activity and bacterial community structure therein. The study was conducted in three stages: (i) cultivation of C. juncea in soil contaminated with 200, 400, and 800 g ha−1 STZ; (ii) determination of the soil microbial activity (basal respiration, microbial biomass carbon, and bacterial community structure); and (iii) cultivation of a bioindicator species and determination of the residual fraction of STZ. The soil microbial activity was impacted by the soil type and STZ dose. Soil previously cultivated with C. juncea (rhizospheric soil) displayed higher CO2 and lower qCO2 values than non-rhizospheric soil (no previous C. juncea cultivation). Increasing doses of STZ reduced the activity and lowered the diversity indices of the soil microorganisms. The bacterial community structure was segregated between the rhizospheric and non-rhizospheric soils. Regardless of soil type, the bioindicator of remediation (Pennisetum glaucum R.Br.) grew only at the STZ dose of 200 g ha−1, and the plant intoxication level was also lower in rhizospheric soil treated with this herbicide dose. All P. glaucum plants died in the soils treated with 400 and 800 g ha−1 STZ. Previous cultivation of C. juncea in soils contaminated with 200, 400, and 800 g ha−1 STZ reduced the residual fraction of the herbicide by 4.8%, 12.5%, and 17.4%, respectively, compared with that in the non-rhizospheric soils. In conclusion, previous cultivation with C. juncea promoted increases in the soil bacterial activity and diversity indices, mitigated the deleterious effects of STZ on the bioindicator crop, and reduced the residual fraction of the herbicide in the soil.





Similar content being viewed by others
Data availability
The authors of the manuscript “Crotalaria juncea enhances the bioremediation of sulfentrazone-contaminated soil and promotes changes in the soil bacterial community” declare that all data generated and analyzed in this study were not submitted for publication in other scientific journals.
References
Ortiz-Hernández ML, Sánchez-Salinas E, Dantán-González E, Castrejón-Godínez ML (2013) Pesticide biodegradation: mechanisms, genetics and strategies to enhance the process. In: Chamy R, Rosenkranz F (eds) Biodegradation – life of science. Intech, Rijeka, pp 251–287. https://doi.org/10.5772/56098
Jacobsen CS, Hjelmsø MH (2014) Agricultural soils, pesticides and microbial diversity. Curr Opin Biotechnol 27:15–20. https://doi.org/10.1016/j.copbio.2013.09.003
Rigotto RM, e Vasconcelos DP, Rocha MM (2014) Pesticide use in Brazil and problems for public health. Cad Saude Publica 30:1360–1362
Ferraço M, Belo AF, Pires FR, Bonomo R, Filho AC (2019) Phytoremediation of contaminated soil with sulfentrazone by different density of crotalaria juncea. Planta Daninha 37. https://doi.org/10.1590/s0100-83582019370100008
IBAMA (2022) Boletins anuais de produção, importação, exportação e vendas de agrotóxicos no Brasil. Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis. http://www.ibama.gov.br/index.php?option=com_content&view=article&id=594. Accessed 10 Oct 2022
Belo AF, Pires FR, Bonomo R, Cargnelutti Filho A, Tenis LHO (2016) Sulfentrazone phytoremediation under field conditions. Revista Caatinga 29:119–126. https://doi.org/10.1590/1983-21252016v29n114rc
Santos E, Pires FR, Ferreira AD, Egreja Filho FB, Madalão JC, Bonomo R, da Rocha Junior PR (2019) Phytoremediation and natural attenuation of sulfentrazone: mineralogy influence of three highly weathered soils. Int J Phytoremediation 21:652–662. https://doi.org/10.1080/15226514.2018.1556583
Martinez CO, Silva CMMS, Fay EF, Abakerli RB, Maia AHN, Durrant LR (2010) Microbial degradation of sulfentrazone in a Brazilian rhodic hapludox soil. Braz J Microbiol 41:209–217. https://doi.org/10.1590/S1517-83822010000100030
Melo CAD, Massenssini AM, Passos ABRJ, Carvalho FP, Ferreira LR, Silva AA, Costa MD (2016) Isolation and characteristics of sulfentrazone-degrading bacteria. J Environ Sci Health B 52:115–121. https://doi.org/10.1080/03601234.2016.1248136
Melo CAD, de Souza WM, de Carvalho FP, Massenssini AM, da Silva AA, Ferreira LR, Costa MD (2017) Microbial activity of soil with sulfentrazone associated with phytoremediator species and inoculation with a bacterial consortium. Bragantia 76:300–310. https://doi.org/10.1590/1678-4499.203
Shaner DL (2012) Field dissipation of Sulfentrazone and Pendimethalin in Colorado, weed. Technology 26:633–637. https://doi.org/10.1614/WT-D-12-00037.1
Madalão JC, Pires FR, Chagas K, Cargnelutti Filho A, Procópio SO (2012) Uso de leguminosas na fitorremediação de solo contaminado com sulfentrazone. Pesqui Agropecu Trop 42:390–396. https://doi.org/10.1590/S1983-40632012000400001
Madalão JC, Pires FR, Cargnelutti Filho A, Nascimento AF, Chagas K, Procopio SO, Araujo RS, Bonomo R, Taufner GA (2012) Selection of species tolerant to the herbicide sulfentrazone with potential for phytoremediation of contaminated soils. Semin Cienc Agrar 33:2199–2214. https://doi.org/10.5433/1679-0359.2012v33n6p2199
Kong Z, Glick BR (2017) The role of plant growth-promoting bacteria in metal phytoremediation. Adv Microb Physiol 71:97–132. https://www.sciencedirect.com/science/article/abs/pii/S0065291117300103
Yaashikaa PR, Kumar PS (2022) Bioremediation of hazardous pollutants from agricultural soils: a sustainable approach for waste management towards urban sustainability. Environ Pollut 312:120031. https://doi.org/10.1016/J.ENVPOL.2022.120031
Hou J, Liu W, Wang B, Wang Q, Luo Y, Franks AE (2015) PGPR enhanced phytoremediation of petroleum contaminated soil and rhizosphere microbial community response. Chemosphere 138:592–598. https://doi.org/10.1016/j.chemosphere.2015.07.025
Pushpanathan M, Jayashree S, Gunasekaran P, Rajendhran J (2014) Microbial bioremediation: a metagenomic approach. In: Microbial biodegradation and bioremediation. Elsevier Inc, pp 408–419. https://doi.org/10.1016/B978-0-12-800021-2.00017-0
Leung KT, Jiang Z-H, Almzene N, Nandakumar K, Sreekumari K, Trevors JT (2019) Biodegradation and bioremediation of organic pollutants in soil. In: van Elsas JD, Trevors JT, Rosado AS, Nannipieri P (eds) Modern soil microbiology, 3rd edn. CRC Press, London, pp 381–402
Martinez CO, Silva CMMS, Fay EF, Abakerli RB, Maia AHN, Durrant LR (2010) Microbial degradation of sulfentrazone in a Brazilian rhodic hapludox soil. Braz J Microbiol 41:209–217. https://doi.org/10.1590/S1517-83822010000100030
de Souza AJ, de Andrade PAM, de Araújo Pereira AP, Andreote FD, Tornisielo VL, Regitano JB (2017) The depleted mineralization of the fungicide chlorothalonil derived from loss in soil microbial diversity. Sci Rep 7. https://doi.org/10.1038/s41598-017-14803-0
Mielke KC, Bertuani RR, Pires FR, Bueno Cotta AJ, Egreja Filho FB, Madalão JC (2020) Does Canavalia ensiformis inoculation with Bradyrhizobium sp. enhance phytoremediation of sulfentrazone-contaminated soil? Chemosphere 255:127033. https://doi.org/10.1016/j.chemosphere.2020.127033
Ferreira LC, de Moreira BRA, Montagnolli RN, Prado EP, da Viana RS, Tomaz RS, Cruz JM, Bidoia ED, Frias YA, Lopes PRM (2021) Green manure species for phytoremediation of soil with tebuthiuron and vinasse. Front Bioeng Biotechnol 8:1380. https://doi.org/10.3389/FBIOE.2020.613642/BIBTEX
Soil Survey Staff (2014) Keys to soil taxonomy, 12th edn. USDA-Natural Resources Conservation Service, Washington https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/survey/class/taxonomy/?cid=nrcs142p2_053580. Accessed 10 Oct 2022
Embrapa (2011) Manual de Métodos de Análises de solo, 2nd edn. Rio de Janeiro
van Raij B, de Andrade JC, Cantarella H, Quaggio JA, van Raij B, de Andrade JC, Cantarella H, Quaggio JA (2001) Análise química para avaliação da fertilidade de solos tropicais. In: Instituto Agronômico de, 1st edn, Campinas, Campinas. www.iac.br
Allef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic Press, London
Islam KR, Weil RR (1998) Microwave irradiation of soil for routine measurement of microbial biomass carbon. Biol Fertil Soils 27:408–416. https://doi.org/10.1007/s003740050451
Vance EDD, Brookes PCC, Jenkinson DSS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. https://doi.org/10.1016/0038-0717(87)90052-6
ALAM (1974) Recomendaciones sobre unificación de los sistemas de evaluación en ensayos de control de malezas – ScienceOpen. https://www.scienceopen.com/document?vid=83f65558-5420-4054-b5c3-1da660ac8346. Accessed 10 Oct 2022
Goulart SM, de Queiroz MELR, Neves AA, de Queiroz JH (2008) Low-temperature clean-up method for the determination of pyrethroids in milk using gas chromatography with electron capture detection. Talanta 75:1320–1323. https://doi.org/10.1016/j.talanta.2008.01.058
Passos ABRJ, Souza MF, Silva DV, Saraiva DT, da Silva AA, Zanuncio JC, Gonçalves BFS (2018) Persistence of picloram in soil with different vegetation managements. Environ Sci Pollut Res 25:23986–23991. https://doi.org/10.1007/S11356-018-2443-Y/TABLES/4
PUBCHEM (2019) National Center for biotechnology information. Sulfentrazone, CID=86369. PubChen Database. https://pubchem.ncbi.nlm.nih.gov/compound/86369. Accessed 8 Jan 2019
FMC, FMC Agrícola. Produtos (2017) https://www.fmcagricola.com.br/produtosdetalhes.aspx?cod=40. Accessed 8 Jan 2017
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700. https://doi.org/10.1128/aem.59.3.695-700.1993
Heuer H, Krsek M, Baker P, Smalla K, Wellington EM (1997) Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol 63:3233–3241. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC168621/
Nübel U, Engelen B, Felske A, Snaidr J, Wieshuber A, Amann RI, Ludwig W, Backhaus H (1996) Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol 178:5636–5643. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC178401/
Kozdroj J, van Elsas JD, Kozdrój J, van Elsas JD (2001) Structural diversity of microorganisms in chemically perturbed soil assessed by molecular and cytochemical approaches. J Microbiol Methods 43:197–212. https://doi.org/10.1016/S0167-7012(00)00197-4
De Araujo Pereira AP, PAM DA, Bini D, Durrer A, Robin A, Bouillet JP, Andreote FD, EJBN C (2017) Shifts in the bacterial community composition along deep soil profiles in monospecific and mixed stands of eucalyptus Grandis and Acacia mangium. PLoS One 12:e0180371. https://doi.org/10.1371/journal.pone.0180371
Hammer DAT, Ryan PD, Hammer Ø, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm
Andreote FD, Azevedo JL, Araújo WL (2009) Assessing the diversity of bacterial communities associated with plants. Braz J Microbiol 40:417–432
Zheng T, Liang C, Xie H, Zhao J, Yan E, Zhou X, Bao X (2019) Rhizosphere effects on soil microbial community structure and enzyme activity in a successional subtropical forest. FEMS Microbiol Ecol 95. https://doi.org/10.1093/FEMSEC/FIZ043
Hakim S, Nawaz MS, Siddique MJ, Hayat M, Gulzar U, Imran A (2022) Metagenomics for rhizosphere engineering. Rhizosphere Engineering:395–416. https://doi.org/10.1016/B978-0-323-89973-4.00022-3
Cycoń M, Mrozik A, Piotrowska-Seget Z (2017) Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: a review. Chemosphere 172:52–71. https://doi.org/10.1016/j.chemosphere.2016.12.129
Gill HK, Garg H (2014) Pesticides: environmental impacts and management strategies. In: Larramendy ML, Soloneski S (eds) Pesticides toxic effects. InTech, Rijeka, pp 187–230
Kafle A, Timilsina A, Gautam A, Adhikari K, Bhattarai A, Aryal N (2022) Phytoremediation: mechanisms, plant selection and enhancement by natural and synthetic agents. Environ Adv 8. https://doi.org/10.1016/j.envadv.2022.100203
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486. https://doi.org/10.1016/j.tplants.2012.04.001
Newman LA, Doty SL, Gery KL, Heilman PE, Muiznieks I, Shang TQ, Siemieniec ST, Strand SE, Wang X, Wilson AM, Gordon MP (2010) Phytoremediation of organic contaminants: a review of phytoremediation research at the University of Washington. J Soil Contam 7:531–542. https://doi.org/10.1080/10588339891334366
Dan HA, Dan LGM, Barroso ALL, Procópio SO, Oliveira RS, Assis RL, Silva AG, Feldkircher C (2011) Atividade residual de herbicidas pré-emergentes aplicados na cultura da soja sobre o milheto cultivado em sucessão. Planta Daninha 29:437–445. https://doi.org/10.1590/S0100-83582011000200022
Pereira APA, de Souza AJ, de Chaves MG, Fracetto GGM, Garcia KGV, Filho PFM, Cardoso EJBN (2021) Mechanisms of the phytomicrobiome for enhancing soil fertility and health. In: New and future developments in microbial biotechnology and bioengineering. Elsevier, pp 1–14. https://doi.org/10.1016/B978-0-444-64325-4.00001-8
Pires FR, Marcianoe Souza C, Cecon PR, Barbosa Dos Santos J, Tótola MR, de Oliveira Procópio S, Da Silva AA, Silva CSW (2005) Inferências sobre atividade rizosférica de espécies com potencial para fitorremediação do herbicida tebuthiuron. Rev Bras Cienc Solo 29:627–634. https://doi.org/10.1590/S0100-06832005000400015
Madalão JC, da Silva AA, de Orlando WA, Saraiva DT, Melo CAD, D’ Antonino L (2016) O herbicida sulfentrazone interfere na biomassa microbiana e na atividade da microbiota do solo, Revista de Ciências Agrarias. Amazon J Agric Environ Sci 59:54–59. https://doi.org/10.4322/rca.2016
Nwachukwu BC, Ayangbenro AS, Babalola OO (2021) Elucidating the rhizosphere associated bacteria for environmental sustainability. Agriculture 11:75. https://doi.org/10.3390/AGRICULTURE11010075
Li E, de Jonge R, Liu C, Jiang H, Friman V-P, Pieterse CMJ, Bakker PAHM, Jousset A (2021) Rapid evolution of bacterial mutualism in the plant rhizosphere. Nat Commun 12:1–13. https://doi.org/10.1038/s41467-021-24005-y
Reis FCD, Tornisielo VL, Martins BAB, Souza AJD, Andrade PAMD, Andreote FD, Silveira RF, Victória Filho R (2019) Respiration induced by substrate and bacteria diversity after application of diuron, hexazinone, and sulfometuron-methyl alone and in mixture. J Environ Sci Health B 54. https://doi.org/10.1080/03601234.2019.1620043
de Souza AJ, Pereira APDA, Andreote FD, Tornisielo VL, Tizioto PC, Coutinho LL, Regitano JB (2021) Sulfadiazine dissipation as a function of soil bacterial diversity. Environ Pollut 271. https://doi.org/10.1016/j.envpol.2020.116374
de Souza AJ, de Araújo Pereira AP, Pedrinho A, Andreote FD, Tornisielo VL, Tizioto PC, Coutinho LL, Regitano JB (2022) Land use and roles of soil bacterial community in the dissipation of atrazine. Sci Total Environ 827. https://doi.org/10.1016/j.scitotenv.2022.154239
Morillo E, Villaverde J (2017) Advanced technologies for the remediation of pesticide-contaminated soils. Sci Total Environ 586:576–597. https://doi.org/10.1016/J.SCITOTENV.2017.02.020
Satish GP, Ashokrao DM, Arun SK (2017) Microbial degradation of pesticide: a review. Afr J Microbiol Res 11:992–1012. https://doi.org/10.5897/ajmr2016.8402
Martinazzo R, Jablonowski ND, Hamacher G, Dick DP, Burauel P (2010) Accelerated degradation of (14)C-atrazine in brazilian soils from different regions. J Agric Food Chem 58:7864–7870. https://doi.org/10.1021/jf100549d
dos Santos EA, Costa MD, Ferreira LR, dos Reis MR, França AC, dos Santos JB (2010) Atividade Rizosférica de Solo Tratado com Herbicida Durante Processo de Remediação por Stizolobium aterrimum. Pesqui Agropecu Trop:1–7 https://revistas.ufg.br/pat/article/view/4670. Accessed 18 Sept 2022
Vieira RF, Silva CMMS, Silveira APD (2007) Soil microbial biomass C and symbiotic processes associated with soybean after sulfentrazone herbicide application. Plant Soil 300:95–103. https://doi.org/10.1007/S11104-007-9392-4/FIGURES/4
Vivian R, Reis MR, Jakelaitis A, Silva AF, Guimarães AA, Santos JB, Silva AA (2006) Persistência de sulfentrazone em Argissolo Vermelho-Amarelo cultivado com cana-de-açúcar. Planta Daninha 24:741–750. https://doi.org/10.1590/S0100-83582006000400015
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as ph, on the microbial biomass of forest soils. Soil Biol Biochem 25:393–395. https://doi.org/10.1016/0038-0717(93)90140-7
Chaer GM, Tótola MR (2007) Impacto do manejo de resíduos orgânicos durante a reforma de plantios de eucalipto sobre indicadores de qualidade do solo. Rev Bras Cienc Solo 31:1381–1396. https://doi.org/10.1590/S0100-06832007000600016
Hartman WH, Richardson CJ (2013) Differential nutrient limitation of soil microbial biomass and metabolic quotients (qCO2): is there a biological stoichiometry of soil microbes? PLoS One 8:e57127. https://doi.org/10.1371/JOURNAL.PONE.0057127
Feld L, Hjelmsø MH, Nielsen MS, Jacobsen AD, Rønn R, Ekelund F, Krogh PH, Strobel BW, Jacobsen CS (2015) Pesticide side effects in an agricultural soil ecosystem as measured by amoA expression quantification and bacterial diversity changes. PLoS One 10:e0126080
Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK (2020) Plant–microbiome interactions: from community assembly to plant health. Nat Rev Microbiol 18:607–621. https://doi.org/10.1038/s41579-020-0412-1
Virk AL, Lin BJ, Kan ZR, Qi JY, Dang YP, Lal R, Zhao X, Zhang HL (2022) Simultaneous effects of legume cultivation on carbon and nitrogen accumulation in soil. Adv Agron 171:75–110. https://doi.org/10.1016/BS.AGRON.2021.08.002
Yu H, Wang F, Shao M, Huang L, Xie Y, Xu Y, Kong L (2021) Effects of rotations with legume on soil functional microbial communities involved in phosphorus transformation. Front Microbiol 12:2629. https://doi.org/10.3389/FMICB.2021.661100/BIBTEX
Volpiano CG, Lisboa BB, de São José JFB, Beneduzi A, Granada CE, Vargas LK (2022) Soil-plant-microbiota interactions to enhance plant growth. Rev Bras Cienc Solo 46. https://doi.org/10.36783/18069657RBCS20210098
Li W, Li Y, Lv J, He X, Wang J, Teng D, Jiang L, Wang H, Lv G (2022) Rhizosphere effect alters the soil microbiome composition and C, N transformation in an arid ecosystem. Appl Soil Ecol 170:104296. https://doi.org/10.1016/J.APSOIL.2021.104296
Maron PA, Sarr A, Kaisermann A, Lévêque J, Mathieu O, Guigue J, Karimi B, Bernard L, Dequiedt S, Terrat S, Chabbi A, Ranjard L (2018) High microbial diversity promotes soil ecosystem functioning. Appl Environ Microbiol 84:AEM.02738–17. https://doi.org/10.1128/AEM.02738-17
Pushpanathan M, Jayashree S, Gunasekaran P, Rajendhran J, Regitano JB, Bonfleur EJ, Ahmad I, Ahmad F, Pichtel J, Santos WF, de Oliveira MS, Cristina L, Leói T, Alisson M, Cunha A, Sarma H, Nava AR, Prasad MNV, Trivedi C et al (2018) Effect of temperature on the dissipation of seven herbicides in a biobed matrix. Sci Total Environ 2:138–140. https://doi.org/10.1111/j.1365-2389.2009.01184.x
Delgado-Baquerizo M, Oliverio AM, Brewer TE, Benavent-gonzález A, Eldridge DJ, Bardgett RD, Maestre FT, Singh BK, Fierer N (1979) Bacteria found in soil. Science 325(2018):320–325. https://doi.org/10.1126/science.aap9516
Acknowledgements
We thank the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil; Finance Code 001) for granting a scholarship to the first author and the National Council for Scientific and Technological Development (CNPQ, Brazil; Process 130616/2017-6) for research support.
Author information
Authors and Affiliations
Contributions
AJS contributed to data analysis and manuscript writing. ES contributed to all essay assemble, data analysis, and manuscript writing. DGV and APAP contributed to data analysis and manuscript revision. FPR contributed to study conception and design and manuscript revision. ISC, FBEF, and KCFS contributed to DNA analyses and manuscript review. In addition, the publication was reviewed, verified, and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Enderson Ferreira
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 15 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Souza, A.J., Santos, E., Ribeiro, F.P. et al. Crotalaria juncea L. enhances the bioremediation of sulfentrazone-contaminated soil and promotes changes in the soil bacterial community. Braz J Microbiol 54, 2319–2331 (2023). https://doi.org/10.1007/s42770-023-01064-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01064-5