Abstract
In 2019, severe acute respiratory syndrome caused by CoV-2 virus became a pandemic worldwide, being the fast spread of the disease due to the movement of infected people from one country to another, from one continent to another, or within the same country. Associated comorbidities are important factors that predispose to any fungal coinfections. Because of the importance of fungal infections in COVID-19 patients, the aim of this work was to collect data of the more encountered mycoses related to patients undergoing this disease. Aspergillosis was the first COVID-19-related fungal infection reported, being A. fumigatus the most frequent species for CAPA. Other fungal infections related include mainly candidiasis and mucormycosis, being Rhizopus spp. the more prevalent species found. Influenza-associated pulmonary aspergillosis is well documented; thus, similar complications are expected in severe forms of COVID-19 pneumonia. Therefore, in patients with COVID-19, it is important to take special attention to the surveillance and suspicion of fungal coinfections that might worsen the patient’s prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2019 in Wuhan, China, cases of unexpected pneumonia have emerged. The etiological agent of the disease that causes severe acute respiratory syndrome (SARS) is a virus belonging to Coronaviridae family, named coronavirus (CoV-2) [1]. The first was reported in 2002 (SARS-CoV) in China and in the Middle East, Saudi Arabia in 2012, (MERS)-CoV. Thus far, SARS-CoV-2 has become a pandemic worldwide, and until now (June 23rd 2021) over 179 million of infected people and over 3 million deaths have been reported representing an obit percentage of 2.1% [2]. The rapid spread of coronavirus disease was largely due to the movement by traveling of infected people from one country to another, from one continent to another, or within the same country.
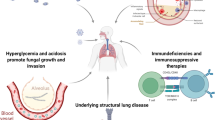
Overall, the disease pattern ranged from asymptomatic, mild flu-like to severe respiratory distress. Associated comorbidities such diabetes, chronic obstructive pulmonary disease, immunocompromised conditions like corticosteroid, interleukin inhibitors or broad-spectrum antibiotic therapy, mechanical ventilation, long-term stay in intensive care unit stay, severe lung tissue damage, acute respiratory distress syndrome, use of catheters, immunological dysfunction, immune dysregulation characterized by decreased T cells including CD4 and CD8 cells, alveolar macrophages activity disturbed, cytokine storm, and lymphocytopenia are often seen [3,4,5,6]. Tomography findings are in mainly consisting of ground-glass opacities, nodular infiltrates and consolidations, bullous emphysema, interstitial change, halo sign, and reverse halo sign similar to what we see in patients with mucormycosis [7,8,9]. Clinical symptoms including cough, fever, dyspnea, and/or respiratory insufficiency are observed among many others [10].
These are indeed predisposing for any fungal coinfection, such as invasive aspergillosis (IA), disseminated candidiasis, endemic mycoses, phaeohyphomycosis, mucormycosis, or fusariosis, among others, even in the absence of classical well-defined host factors [6, 10, 11]. Prolonged use of corticosteroids is considered a risk factor for invasive fungal diseases [12]. The relation between COVID-19 and aspergillosis is known as CAPA (COVID-19-associated pulmonary aspergillosis). The criteria to classify patients with CAPA vary from those with risk factors for an IPA (invasive pulmonary aspergillosis), as considered by the EORTC [13], or those with other factors such as diabetes, obesity, or hypertension. Criteria based on AspICU algorithm [14] were also applied. Recently, a case definition for IAPA (influenza-associated pulmonary aspergillosis) was proposed by an expert panel to classify patients with CAPA, classified as putative aspergillosis rather than probable or proven [6]. In the IAPA case definition, host factors are not used because IAPA may develop in any patient with severe influenza. The European Society for Clinical Microbiology and International Society of Human and Animal Mycology recently published guidance to identify proven, probable, and possible CAPA [15]. Regardless of the definition, it is difficult to distinguish between infection and colonization.
It was reported that 14–30% of hospitalized patients diagnosed with COVID-19 develop a severe respiratory failure requiring intensive care admission. Invasive aspergillosis appeared at a range from 11 to 21 days after the onset of COVID-19 and affected up to 30% of intubated patients [16,17,18]. Some authors pointed to a mortality rate ranging from 15 to 30%, with less survival in patients with CAPA, compared with those without [1, 19,20,21,22,23,24]. The same trend has been previously observed for influenza-related aspergillosis [25].
In general serum galactomannan (GM) is negative, whereas bronchoalveolar lavage (BAL) GM antigen is reported positive at a percentage of 77.8, being hypothesized that patients have an airway invasive infection rather blood vessels invasion to cause release of galactomannan. Thus, wherever possible BAL GM should be performed, since it is more sensitive than in serum. However, due the high aerosolized risk, this procedure is avoided in COVID-19 patients, and tracheal secretions, for instance, are preferred [6, 15, 20, 26]. In one study, it was observed that Aspergillus tests from COVID-19 patients were similar to those with pneumococcal pneumonia but lower than those with influenza. Thus, it was concluded that in ICU, the specificity of tests is low and tests like pan-fungal B-D glucan should not be used, advising that a positive test for Aspergillus in COVID-19 should be interpreted with caution [27].
COVID-19-associated mucormycosis will be further discussed. This is an infection that is worldwide distributed and has no predilection for a particular country. The large number of reported cases from India is link to possess a high burden with 77 million people with diabetes and another 36.5 million with prediabetes which are a high-risk condition, worsen, if is being uncontrolled, for suffering, particularly, rhino-orbital-cerebral mucormycosis [28, 29].
Treatment with interleukin inhibitors or tocilizumab (monoclonal antibody), which was used as therapy in COVID patients [30, 31], could also potentially increase the risk of other fungal infections, such those, among others, caused by Candida spp., Histoplasma spp., or Pneumocystis jirovecii [32]. Candidemia has been reported in 2.5–6.9% of COVID-19 patients in the ICU, mainly catheter-related infections and often with unfavorable outcomes [33, 34].
Considering all, the objective of this work was to collect data on the more encountered mycoses related to patients undergoing COVID-19. Although there are reviews related to this topic, all are, in general, treated separately. Thus, an updated data considering this information altogether can be found in this single review.
Methods
We searched in Pub Med and Google Scholar database for eligible studies published until May 31th 2021 for COVID-19-related fungal infections, using the key words “COVID-19” AND, “CAPA,” “COVID-19” AND “fungal infections,” “COVID-19 pneumonia,” “COVID-19” AND “Candida” OR candidiasis, “COVID-19” AND “Aspergillus” OR aspergillosis, “COVID-19” AND “Mucormycosis” OR Post “COVID-19” fungal infections, “COVID-19” AND “Cryptococcus,” “COVID-19” AND “Pneumocystis,” “COVID-19” AND “Histoplasma” OR Histoplasmosis OR endemic mycosis. A total of 160 articles were identified through the initial database search. We excluded 32 articles including four reviews, two research letters, and the remain ones for lack of the information we needed or publications that did not report primary data. After the removal of duplicated items and screening based on title and abstract, 134 articles were assessed for eligibility. Sixty-three were related to COVID-19 and invasive pulmonary aspergillosis and definitions of probable, proven, or putative according with the author definition criteria selection, including 36 articles of clinical case description of CAPA related. Thirty-seven publications were related to cases of mucormycosis and twenty-six to other fungal infections, including candidiasis, yeast non-Candida infections, pneumocystosis, and endemic mycosis. We collected data on epidemiology (age, gender, comorbidities), diagnostic methods, fungi isolated, antifungal indicated therapy, and clinical outcomes that are presented in the corresponding tables. We restricted our search to works published in the English language.
Results
Overall, 178 cases of CAPA were published. Mortality was reported in 88 cases; survivors in 80 and 10 were not specified. The main comorbidities reported were DBT, ATH, obesity, and COPD. A total of 163 Aspergillus species were recovered distributed as follows: A. fumigatus (130), following by A. flavus (15), A. niger (5), A. terreus (4), A. nidulans (2,) and one strain of A. ochraceus, A. calidoustus, A. awamorii, A. citrinoterreus, and A. penicilloides. A. fumigatus was generally susceptible to all drugs, except in 3 reports in which the TR34L98H resistance mutation in the cyp51A gene was found, associated with azole resistant [35,36,37]. Voriconazole was the drug most used, following by amphotericin. All data is detailed in Table 1.
Mucormycosis reported cases were 158, mainly related to uncontrolled diabetes. The isolation from different samples includes Mucor spp. (4), Rhizopus spp. (16), R. oryzae (4), R. azygosporus (1), R. arrhizus (1), R. microsporus (4) Lichtheimia spp. (2), and L. ramosa (1). In cases diagnosed by histology only or those from which no isolation from culture was available, it was named as mucormycosis or Mucorales (125). Forty-eight deaths and eighty-two survivors were reported. Amphotericin liposomal formulation and deoxycholate were the most antifungal drugs used. All data is detailed in Table 2.
Fungemia due to Candida species was reported in 149 cases. The mortality was high, but not accurate percentage could be calculated, due non-reported data in 17 cases. Forty-four patients died and 23 survived. The most frequent species isolated from blood cultures were C. albicans (64), C. auris (51), C. glabrata (17), C. tropicalis (9), C. parapsilosis (6), C. dubliniensis (6), C. orthopsilosis (1), and C. krusei (renamed as Pichia kudriavzevii) (2). Non-Candida yeasts seen were Trichosporon asahii (6), Saccharomyces cerevisiae (2), Rhodotorula mucilaginosa (1), and C. neoformans (2). Histoplasmosis, coccidioidomycosis and paracoccidioidomycosis cases were 4, 2, and, 1, respectively. P. jirovecii was reported in two cases. All data is detailed in Table 3.
Only one case of pulmonary fusariosis classified as putative, due by F. proliferatum in an immunocompetent patient, was reported [112]. There are six reports in which mix isolations were described. One describes a pulmonary mucormycosis diagnosed by biopsy, in which from bronchoalveolar lavage A. flavus, A. niger, C. albicans, C. glabrata, and C. krusei were found [113]. In another study, R. arrhizus plus A. fumigatus were isolated from a lung of a patient suffering from COVID-19 [114]. In a patient with a history of pulmonary embolism treated with corticosteroids, R. microsporus plus A. fumigatus were found [42]. In one patient with lymphoma, R. microsporus plus A. fumigatus were isolated from bronchoalveolar lavage [115]. Other report showed in a patient with diabetes and leukemia, A. fumigatus isolated from BAL, and after some days, isolation of R. microsporus was detected, and in other patient with no underlying disease, treated with corticosteroids, A. fumigatus was first isolated and days after L. ramosa [81]. Other report of fatal COVID-19-associated pulmonary aspergillosis described a mix fungi isolation from respiratory tract secretions. A. niger plus C. albicans were isolated from a patient with diabetes, hepatitis B, and hypertension, whereas A. terreus plus C. albicans were isolated from an otherwise healthy patient [116].
Discussion
The use of steroids, such as dexamethasone to modulate immune-mediated organ damage, interleukin inhibitors, and broad-spectrum antibiotics for the management of COVID-19, could exacerbate preexisting comorbidities and enhance the chances of new onset of fungal infections as was above discussed. Due to the high incidence of influenza-associated pulmonary aspergillosis, it seems natural to expect similar complications in severe forms of COVID-19 pneumonia. The incidence of fungal infections in SARS 2003 was 14.8–33% and the mortality rate 25–73.7% [117]. Besides, reports of severe influenza pneumonia complicated by fungal infections were published [118].
It is important to mention that the development of any fungal coinfection is highly expected in colonized patients, given the characteristics of the coronavirus disease. Therefore, taking into consideration, previous risk factors seem necessary, indicating whether coinfections might worsen the patient’s prognostic values. Mortality in patients with COVID-19 and CAPA has been seen to increase compared to COVID-19 patients without CAPA [19]. The high mortality in CAPA patients could be related with critically ill COVID-19 individuals that require mechanical ventilation, who were mostly elderly and had significant co-existing chronic comorbidities [116].
Despite all this, the mortality rate is also high in non-COVID-19 patients at risk such as those with underlying neutropenia with IPA, if treatment is not initiated on time or whether the underlying disease conditions do not improve [119]. Thus, it could be reasonable that an adequate treatment for COVID-19 could have a positive impact on the absence of improvement in the evolution of IPA. Patients with COVID have chronic obstructive pulmonary disease (COPD) for which the association with aspergillosis is well known or asthma/corticoid therapy that are also known risk factors for Aspergillus colonization. Thus, COVID-19 might be a risk factor for aspergillosis, and the underlying pulmonary conditions may favor COVID-19-associated aspergillosis [120]. Corticosteroids treatment, as is indicated in severe COVID-19 patients, increases 3 times the risk to develop invasive fungal infections in comparison to other patients who do not receive steroids [121]. Some reports highlight the need to monitor pneumatoceles that might predispose to pneumothorax and/or cavitary lesions that could be complicated with coinfections like aspergillosis, even in the recovery phase of COVID-19 [57, 122].
Other fungal infection such as candidiasis could be expected due the aforementioned conditions that predispose for suffering a fungemia, being an important issue to be considered. Diseases such as diabetes or severe COVID-19 seem to alter the intestinal barrier function that facilitates Candida translocation, allowing the gut microbiota like Candida species, to reach the bloodstream and then disseminate systemically [123]. The estimated mortality due to invasive candidiasis is 19–40% [124], being even higher among ICU patients, around 70% [125]. Cases of fungemia due to C. albicans and non albicans in COVID patients were reported in several publications. The reported cases of C. auris sound alarming, due the association of COVID-19 with an emerging pan-resistant yeast [34]. However, its sensitivity to antifungal agents should be studied and suspected according to the epidemiological setting. In Brazil, all C. auris were reported as susceptible to azoles, amphotericin, and echinocandins [95]. Some cases have been seen that appeared in colonized patients when they moved from non-COVID-19 rooms to COVID-19 rooms [97]. However, in a report by the CDC evaluating strains originating worldwide, more than 70% of the C. auris isolates were resistant to fluconazole. In the USA, resistance of C. auris isolates was about 90% for fluconazole, 30% for amphotericin B, and less than 5% for echinocandins. These proportions may include multiple isolates from the same individuals and may change as more isolates are tested [126].
No least is the report of C. glabrata pan-echinocandin resistant infection [92]. In Colombia, fungemia due to non-C. albicans was 78.94%, including C. auris [98]. In India, a high percentage of C. auris isolated from blood were resistant to fluconazole, voriconazole, flucytosine, and amphotericin [34]. In a study performed in Minas Gerais, Brazil, from 212 patients, Candida species were isolated in 98.2%, mostly from tracheal aspirate. The authors described a mortality rate of 90.5% and 76.3% in cases related to Candida non-albicans and C. albicans, respectively [91]. Candida was also related to oropharyngeal candidiasis (OPC), infecting old people with cardiovascular diseases and diabetes due to the weaker immune functions of these patients. CoV-2 as HIV virus produce T lymphocytes consumption [40]. Besides, elderly patients have lower activity levels of protective salivary innate defenses [127]. Fungemia by other yeasts such Trichosporon and Saccharomyces cerevisiae/boulardii was reported [98, 103]. This last is used in ICU patients as a probiotic for treatment of diarrheal disorders [128].
Moreover, it is not surprising to isolate Mucorales, since many patients with COVID-19 suffer from diabetes mellitus as their underlying disease that alters the body’s immunological response enhancing fungal proliferation and diminishing the phagocytic capacity of host immune cells [129]. Besides, corticosteroids have other side effects such as blunting the action of insulin with the increment of blood glucose. This hyperglycemic effect is magnified in diabetic patients and can lead to ketoacidosis [130]. In addition, the ketone reductase enzyme in Rhizopus organisms allowing them to thrive in high glucose and acidic conditions, being the reason for the stimulated growth of these organisms in those patients [131]. It is known that in patients with ketoacidosis, rhino-orbital-cerebral mucormycosis can develop, regardless of whether the patient is undergoing a COVID-19 infection. Mucormycosis without concomitant COVID-19 infection has a mortality rate ranging from 40 to 80% [9]. Severe immunocompromised from untreated diabetes made the patients be susceptible to contract both mucormycosis and COVID-19 [4, 61]. In general, uncontrolled diabetes was the main risk factor [67]. The mortality rate appears to reach 100% when both diseases are associated [113]. Besides, it is very important that ophthalmologists suspect the possible orbital infarction syndrome secondary to mucormycosis in these patients [69]. In one report, loss of vision was observed in 66% and orbital exenteration in 38% of the patients analyzed [28].
However, some cases of patients with diabetes without ketosis are reported [29, 70], as well some with non-underlying condition, suggesting a COVID-19 as risk factor due to steroids or interleukin inhibitor therapies [73, 83]. It is important to note that although corticosteroid therapy helps for the treatment of the severe form of COVID, when comorbidities such as diabetes or other immunosuppression factors exit, can be harmful. Steroids can exacerbate hyperglycemia. Therefore, close monitoring hospitalized patients and after discharged should be take into account for possible complications of post-COVID-19 fungal infections such the cases of mucormycosis that have been described [70, 71, 82, 85]. Thus, diagnosis of mucormycosis requires clinical observations, images, histopathologic findings, fungal culture, and surgical debridement which seem to improve patient survival. However, no growth happens very frequently, being important to consider the proper clinical context for suspicion. The risk of Pneumocystis pneumonia increases significantly with severe CD4 lymphocytopenia [132]. This is the case of HIV patients, and also this scenario occurs with COVID-19 infection. Thus, patients without other underlying factors might suffer of pneumocystosis as has been reported [111].
SARS-CoV-2 and endemic mycoses have overlapping risk factors. Coccidioidomycosis, histoplasmosis, and paracoccidioidomycosis have been reported. There is a lack of information if severity of COVID and endemic mycosis can be influenced one by the other. It is possible that the fungus stays in a latent stage and be reactivated due to coronavirus disease related to immune dysregulation. In areas where these fungi are endemic, awareness should be taken [102, 104, 107, 108].
The frequency of COVID-19 in AIDS is not higher than the frequency of COVID-19 in the general population [133, 134]. There are reported cases of COF or other infections in HIV/COVID-19-positive patients. However, we do not think it is unexpected, since patients with CD4 < 100 generally present COF to different Candida species, endemic diseases, or cryptococcosis. We think that these are HIV-positive patients, with low CD4s susceptible for suffering marker diseases and who were infected with COVID-19. This thought is in agreement with the favorable outcome of HIV/COVID/histoplasmosis case patients in whom not lung damage was observed and no ICU was required [108]. The course and presentation of the reported cases do not vary from those negative COVID-19. Those cases should be taken into account to indicate the appropriate treatment but might not be taken as a separate entity. Nevertheless, the true role of the SARS-CoV-2 virus in HIV patients remains to be fully elucidated.
Conclusion
This review aimed to summarize all the main published reports of COVID-19-associated fungal infections identified by different methodologies, among which A. fumigatus can be considered the most prevalent species reported for CAPA. However, it is difficult to compare the different published studies since not all medical centers use the same criteria to define CAPA, reason for which is needed to find consensus on these definitions. The diagnosis is complicated because serum GM is generally negative, with BAL being the most sensitive sample, but it is difficult to perform due to the risk it represents. Cultures are not very profitable either and PCR is not always useful or available. The suspicion and searching for fungal infections, whether of yeast, hyaline, or pigmented fungi, should be taken into account to indicate the appropriate treatment and improve the patient’s prognosis. In addition, it is of paramount importance to make physicians aware of the fact that invasive fungal infections might occur after patients with COVID-19 have been discharged, particularly those with predisposing conditions, such uncontrolled diabetes related with mucormycosis. This entity has been relevant in recent days, due to the indiscriminate increase in reported cases, especially in India. Therefore, it is mandatory to establish an exhaustive patient follow-up and combine different methodologies of laboratory diagnosis, images, and clinical suspicion related to any fungal infection-COVID-19 related.
Availability of data and material
All data is public available.
Code availability
Not applicable for this section.
References
Wang J, Yang Q, Zhang P, Sheng J, Zhou J, Qu T (2020) Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: a retrospective case series. Crit Care 24:299. https://doi.org/10.1186/s13054-020-03046-7
World Health Organization. https://covid19.who.int/ [Accessed 24 June 2021]
Du Z, Xu X, Wu Y, Wang L, Cowling BJ, Meyers LA (2020) Serial interval of COVID-19 among publicly reported confirmed cases. Emerg Infect Dis 26:1341–1343. https://doi.org/10.3201/eid2606.200357
Mehta S, Pandey A (2020) Rhino-orbital mucormycosis associated with COVID-19. Cureus 12:e10726. https://doi.org/10.7759/cureus.10726
Abolghasemi S, Hakamifard A, Sharifynia S, Pourabdollah Toutkaboni M, Azhdari TH (2021) Fatal invasive pulmonary aspergillosis in an immunocompetent patient with COVID-19 due to Aspergillus terreus: a case study. Clin Case Rep 9:2414–2418. https://doi.org/10.1002/ccr3.4051
Verweij PE, Rijnders BJA, Brüggemann RJM, Azoulay E, Bassetti M, Blot S et al (2020) Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med 46:1524–1535. https://doi.org/10.1007/s00134-020-06091-6
García Clemente M, HermidaValverde T, Leizaola-Irigoyen O, Rodríguez AIE, Guillén MA, TelentiAsensio M et al (2021) Can SARS-CoV-2 be a risk factor for pulmonary aspergillosis? Arch Bronconeumol 57(Suppl1):72–3. https://doi.org/10.1016/j.arbres.2020.06.028
Apostolopoulou A, Esquer Garrigos Z, Vijayvargiya P, Lerner AH, Farmakiotis D (2020) Invasive pulmonary aspergillosis in patients with SARS-CoV-2 infection: a systematic review of the literature. Diagnostics (Basel, Switzerland) 10:807. https://doi.org/10.3390/diagnostics10100807
Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B et al (2019) Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19:e405–e421. https://doi.org/10.1016/S1473-3099(19)30312-3
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H et al (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8:475–481. https://doi.org/10.1016/S2213-2600(20)30079-5
Salehi M, Khajavirad N, Seifi A, Salahshour F, Jahanbin B, Kazemizadeh H et al (2021) Proven Aspergillus flavus pulmonary aspergillosis in a COVID-19 patient: a case report and review of the literature. Mycoses 00:1–8. https://doi.org/10.1111/myc.13255
Arastehfar A, Carvalho A, van de Veerdonk FL, Jenks JD, Koehler P, Krause R et al (2020) COVID-19 associated pulmonary aspergillosis (CAPA)-from immunology to treatment. J Fungi 6:91. https://doi.org/10.3390/jof6020091
Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE et al (2020) Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 71:1367–1376. https://doi.org/10.1093/cid/ciz1008
Blot SI, Taccone FS, Van den Abeele A-M, Bulpa P, Meersseman W, Brusselaers N et al (2012) A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med 186:56–64. https://doi.org/10.1164/rccm.201111-1978OC
Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Lopes Colombo A, Hoenigl M et al (2020) Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 21:149–242. https://doi.org/10.1016/S1473-3099(20)30847-1
Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G et al (2020) Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy. Italy JAMA Intern Med 180:1345–1355. https://doi.org/10.1001/jamainternmed.2020.3539
Koehler P, Cornely OA, Böttiger BW, Dusse F, Eichenauer DA, Fuchs F et al (2020) COVID-19 associated pulmonary aspergillosis. Mycoses 63:528–534. https://doi.org/10.1111/myc.13096
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW et al (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA 323:2052–2059. https://doi.org/10.1001/jama.2020.6775
Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis 2020; published online July 28. https://doi.org/10.1093/cid/ciaa1065
Machado M, Valerio M, Álvarez-Uría A, Olmedo M, Veintimilla C, Padilla B et al (2021) Invasive pulmonary aspergillosis in the COVID-19 era: an expected new entity. Mycoses 64:132–143. https://doi.org/10.1111/myc.13213
van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG (2020) COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med 202:132–135. https://doi.org/10.1164/rccm.202004-1038LE
Gangneux J-P, Reizine F, Guegan H, Pinceaux K, Le Balch P, Prat E et al (2020) Is the COVID-19 pandemic a good time to include Aspergillus molecular detection to categorize aspergillosis in ICU patients? A Monocentric Experience J Fungi 6:105. https://doi.org/10.3390/jof6030105
He Y, Li W, Wang Z, Chen H, Tian L, Liu D (2020) Nosocomial infection among patients with COVID-19: a retrospective data analysis of 918 cases from a single center in Wuhan. China Infect Control Hosp Epidemiol 41:982–983. https://doi.org/10.1017/ice.2020.126
van Grootveld R, van Paassen J, de Boer MGJ, Claas ECJ, Kuijper EJ, van der Beek MT et al (2021) Systematic screening for COVID-19 associated invasive aspergillosis in ICU patients by culture and PCR on tracheal aspirate. Mycoses 64:641–650. https://doi.org/10.1111/myc.13259
Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, Lagrou K et al (2018) Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med 6:782–792. https://doi.org/10.1016/S2213-2600(18)30274-1
Sharma A, Hofmeyr A, Bansal A, Thakkar D, Lam L, Harrington Z et al (2021) COVID-19 associated pulmonary aspergillosis (CAPA): an Australian case report. Med Mycol Case Rep 31:6–10. https://doi.org/10.1016/j.mmcr.2020.06.002
Yusuf E, Vonk A, van den Akker JPC, Bode L, Sips GJ, Rijnders BJA et al (2021) Frequency of positive Aspergillus tests in COVID-19 patients in comparison to other patients with pulmonary infections admitted to the Intensive Care Unit. J Clin Microbiol 59:e02278-e2320. https://doi.org/10.1128/JCM.02278-20
Moorthy A, Gaikwad R, Krishna S, Hegde R, Tripathi KK, Kale PG, et al. (2021) SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg:1–8. https://doi.org/10.1007/s12663-021-01532-1
Revannavar SM, Supriya PS, Samaga L, Vineeth VK (2021) COVID-19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep 14:e241663. https://doi.org/10.1136/bcr-2021-241663
Uchida T, Okamoto M, Fujikawa K, Yoshikawa D, Mizokami A, Mihara T et al (2019) Gastric mucormycosis complicated by a gastropleural fistula: a case report and review of the literature. Medicine (Baltimore) 98:e18142. https://doi.org/10.1097/MD.0000000000018142
Honda H, Kida H, Yoshida M, Fujii M, Ihara S, Goya S et al (2011) Recurrent allergic bronchopulmonary aspergillosis in a patient with rheumatoid arthritis treated with etanercept and tocilizumab. Mod Rheumatol 21:660–664. https://doi.org/10.1007/s10165-011-0449-0
Vallabhaneni S, Chiller TM (2016) Fungal infections and new biologic therapies. Curr Rheumatol Rep 18:29. https://doi.org/10.1007/s11926-016-0572-1
Antinori S, Bonazzetti C, Gubertini G, Capetti A, Pagani C, Morena V et al (2020) Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun Rev 19:102564. https://doi.org/10.1016/j.autrev.2020.102564
Chowdhary A, Tarai B, Singh A, Sharma A (2020) Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April-July 2020. Emerg Infect Dis 26:2694–2696. https://doi.org/10.3201/eid2611.203504
Mohamed A, Hassan T, Trzos-Grzybowska M, Thomas J, Quinn A, O’Sullivan M et al (2021) Multi-triazole-resistant Aspergillus fumigatus and SARS-CoV-2 co-infection: A lethal combination. Med Mycol Case Rep 31:11–14. https://doi.org/10.1016/j.mmcr.2020.06.005
Meijer EFJ, Dofferhoff ASM, Hoiting O, Buil JB, Meis JF (2020) Azole-resistant COVID-19-associated pulmonary aspergillosis in an immunocompetent host: a case report. J Fungi 6:79. https://doi.org/10.3390/jof6020079
Ghelfenstein-Ferreira T, Saade A, Alanio A, Bretagne S, Araujo de Castro R, Hamane S et al (2021) Recovery of a triazole-resistant Aspergillus fumigatus in respiratory specimen of COVID-19 patient in ICU - A case report. Med Mycol Case Rep 31:15–8. https://doi.org/10.1016/j.mmcr.2020.06.006
Fernandez NB, Caceres DH, Beer KD, Irrazabal C, Delgado G, Farias L et al (2021) Ventilator-associated pneumonia involving Aspergillus flavus in a patient with coronavirus disease 2019 (COVID-19) from Argentina. Med Mycol Case Rep 31:19–23. https://doi.org/10.1016/j.mmcr.2020.07.001
Lescure F-X, Bouadma L, Nguyen D, Parisey M, Wicky P-H, Behillil S et al (2020) Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis 20:697–706. https://doi.org/10.1016/S1473-3099(20)30200-0
Salehi M, Ahmadikia K, Mahmoudi S, Kalantari S, Jamalimoghadamsiahkali S, Izadi A et al (2020) Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses 63:771–778. https://doi.org/10.1111/myc.13137
Benedetti MF, Alava KH, Sagardia J, Corella Cadena R, Laplume D, Capece P et al (2021) COVID-19 associated pulmonary aspergillosis in ICU patients: report of five cases from Argentina. Med Mycol Case Rep 31:24–28. https://doi.org/10.1016/j.mmcr.2020.11.003
Sasoni N, Rodriguez Müller M, Posse G, González J, Leonardelli F, Garcia-Effron G (2021) SARS-CoV-2 and Aspergillus section Fumigati coinfection in an immunocompetent patient treated with corticosteroids. Rev Iberoam Micol 38:16–18. https://doi.org/10.1016/j.riam.2020.11.001
Prattes J, Valentin T, Hoenigl M, Talakic E, Reisinger AC, Eller P (2021) Invasive pulmonary aspergillosis complicating COVID-19 in the ICU - a case report. Med Mycol Case Rep 31:2–5. https://doi.org/10.1016/j.mmcr.2020.05.001
Rutsaert L, Steinfort N, Van Hunsel T, Bomans P, Naesens R, Mertes H et al (2020) COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care 10:71. https://doi.org/10.1186/s13613-020-00686-4
Wu S, Yang S, Chen R, Chen H, Xu Y, Lin B (2020) Dynamic immune response profiles and recovery of a covid-19 patient with coinfection of Aspergillus fumigatus and other baseline diseases: A case report. OMICS 24:615–618. https://doi.org/10.1089/omi.2020.0110
Helleberg M, Steensen M, Arendrup MC (2021) Invasive aspergillosis in patients with severe COVID-19 pneumonia. Clin Microbiol Infect 27:147–148. https://doi.org/10.1016/j.cmi.2020.07.047
Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B (2020) Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med 8:e48–e49. https://doi.org/10.1016/S2213-2600(20)30237-X
Blaize M, Mayaux J, Nabet C, Lampros A, Marcelin A-G, Thellier M et al (2020) Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis 26:1636–1637. https://doi.org/10.3201/eid2607.201603
Dupont D, Menotti J, Turc J, Miossec C, Wallet F, Richard J-C et al (2021) Pulmonary aspergillosis in critically ill patients with coronavirus disease 2019 (COVID-19). Med Mycol 59:110–114. https://doi.org/10.1093/mmy/myaa078
Lahmer T, Kriescher S, Herner A, Rothe K, Spinner CD, Schneider J et al (2021) Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: results from the prospective AspCOVID-19 study. PLoS ONE 16:e0238825. https://doi.org/10.1371/journal.pone.0238825
Van Biesen S, Kwa D, Bosman RJ, Juffermans NP (2020) Detection of invasive pulmonary aspergillosis in covid-19 with non-directed bronchoalveolar lavage. Am J Respir Crit Care Med 202:1171–1173. https://doi.org/10.1164/rccm.202005-2018LE
Nasir N, Farooqi J, Mahmood SF, Jabeen K (2020) COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses 63:766–770. https://doi.org/10.1111/myc.13135
Falces-Romero I, Ruiz-Bastián M, Díaz-Pollán B, Maseda E, García-Rodríguez J (2020) SARS-CoV-2 Working Group. Isolation of Aspergillus spp. in respiratory samples of patients with COVID-19 in a Spanish Tertiary Care Hospital. Mycoses 63:1144–48. https://doi.org/10.1111/myc.13155
Martín CS, Martíne EM, Pellicer RG, Ibáñez RA, González EM, Pitarch JVL. Invasive pulmonary aspergillosis in patients with acute respiratory syndrome by COVID-19. Rev Esp Anestesiol Reanim 2021; published online April 20. https://doi.org/10.1016/j.redar.2021.02.012
Lamoth F, Glampedakis E, Boillat-Blanco N, Oddo M, Pagani J-L (2020) Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin Microbiol Infect 26:1706–1708. https://doi.org/10.1016/j.cmi.2020.07.010
Mitaka H, Perlman DC, Javaid W, Salomon N (2020) Putative invasive pulmonary aspergillosis in critically ill patients with COVID-19: an observational study from New York City. Mycoses 63:1368–1372. https://doi.org/10.1111/myc.13185
Mallappa M, Rangappa P, Jacob I, Helavar RV, Rao K (2021) A case report of coronavirus disease-19-associated pulmonary aspergillosis and pneumothorax. Indian J Case Reports 7:146–148. https://doi.org/10.32677/IJCR.2021.v07.i04.007
Trovato L, Calvo M, Migliorisi G, Astuto M, Oliveri F, Oliveri S (2021) Fatal VAP-related pulmonary aspergillosis by Aspergillus niger in a positive COVID-19 patient. Respir Med case reports 32:101367. https://doi.org/10.1016/j.rmcr.2021.101367
Hakamifard A, Hashemi M, Fakhim H, Aboutalebian S, Hajiahmadi S, Mohammadi R (2021) Fatal disseminated aspergillosis in an immunocompetent patient with COVID-19 due to Aspergillus ochraceus. J Mycol Med 31:101124. https://doi.org/10.1016/j.mycmed.2021.101124
Santana MF, Pivoto G, Alexandre MAA, Baía-da-Silva DC, da Silva Borba MG, Almeida Val F et al (2020) Confirmed invasive pulmonary aspergillosis and COVID-19: the value of postmortem findings to support antemortem management. Rev Soc Bras Med Trop 53:e20200401. https://doi.org/10.1590/0037-8682-0401-2020
Werthman-Ehrenreich A (2021) Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med 42:264.e5-264.e8. https://doi.org/10.1016/j.ajem.2020.09.032
Alekseyev K, Didenko L, Chaudhry B (2021) Rhinocerebral mucormycosis and COVID-19 pneumonia. J Med Cases 12:85–9. https://doi.org/10.14740/jmc3637
Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Rezaei Kanavi M, Farjad R. Rhino-orbital mucormycosis during steroid therapy in COVID-19 patients: a case report. Eur J Ophthalmol 2021:11206721211009450. https://doi.org/10.1177/11206721211009450
Saldanha M, Reddy R, Vincent MJ. Title of the article: paranasal mucormycosis in COVID-19 patient. Indian J Otolaryngol Head Neck Surg 2021:1–4. https://doi.org/10.1007/s12070-021-02574-0
Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS et al (2020) Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. The Lancet Microbe 1:e245–e253. https://doi.org/10.1016/S2666-5247(20)30115-4
do Monte Junior ES, Dos Santos MEL, Ribeiro IB, de Oliveira Luz G, Ryokababa E, Salomãohirsch B et al (2020) Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin Endosc 2020(53):746–9. https://doi.org/10.5946/ce.2020.180
Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD (2021) Rise of the phoenix: mucormycosis in COVID-19 times. Indian J Ophthalmol 69:1563–1568. https://doi.org/10.4103/ijo.IJO_310_21
Sarkar S, Gokhale T, Choudhury SS, Deb AK (2021) COVID-19 and orbital mucormycosis. Indian J Ophthalmol 69:1002–1004. https://doi.org/10.4103/ijo.IJO_3763_20
Rao R, Shetty AP, Nagesh CP (2021) Orbital infarction syndrome secondary to rhino-orbital mucormycosis in a case of COVID-19: clinico-radiological features. Indian J Ophthalmol 69:1627–1630. https://doi.org/10.4103/ijo.IJO_1053_21
Raghavendra Rao MV, Kumar Chennamchetty V, Adimulapu S, Patel Kola B, De Padua MCA (2021) Post-COVID pulmonary mucormycosis- a case report. IP Indian J Immunol Respir Med 6:62–6. https://doi.org/10.18231/j.ijirm.2021.014
Sharma S, Grover M, Bhargava S, Samdani S, Kataria T (2021) Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol 135:1–6. https://doi.org/10.1017/S0022215121000992
Deshmukh R, Upadhyay K, Patwadkar R, Patil S (2020) Mucor Mycosis in COVID-19: Case reports. J Adv Res Med 7:20–3. https://doi.org/10.24321/2349.7181.202016
Karimi-Galougahi M, Arastou S, Haseli S (2021) Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19). Int Forum Allergy Rhinol 11:1029–1030. https://doi.org/10.1002/alr.22785
Ministerio de salud de Argentina. https://www.argentina.gob.ar/noticias/salud-informa-sobre-un-caso-notificado-de-hongo-negro-asociado-covid-19 [Accessed 22 June 2021]
Pasero D, Sanna S, Liperi C, Piredda D, Branca GP, Casadio L, et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis. Infection 2020; published online Dec 17. https://doi.org/10.1007/s15010-020-01561-x
Mekonnen ZK, Ashraf DC, Jankowski T, Grob SR, Vagefi MR, Kersten RC et al (2021) Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthal Plast Reconstr Surg 37:e40-80. https://doi.org/10.1097/IOP.0000000000001889
Placik DA, Taylor WL, Wnuk NM (2020) Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol Case Reports 15:2378–2381. https://doi.org/10.1016/j.radcr.2020.09.026
Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A et al (2021) Coronavirus disease (covid-19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia 186:289–298. https://doi.org/10.1007/s11046-021-00528-2
Zurl C, Hoenigl M, Schulz E, Hatzl S, Gorkiewicz G, Krause R et al (2021) Autopsy proven pulmonary mucormycosis due to Rhizopus microsporus in a critically Ill COVID-19 patient with underlying hematological malignancy. J Fungi 7:88. https://doi.org/10.3390/jof7020088
Buil JB, van Zanten ARH, Bentvelsen RG, Ripstra TA, Goorhuis B, van der Voort S et al (2021) Case series of four secondary mucormycosis infections in COVID-19 patients, the Netherlands, December 2020 to May 2021. Euro Surveill 26:2100510. https://doi.org/10.2807/1560-7917.ES.2021.26.23.2100510
Dallalzadeh LO, Ozzello DJ, Liu CY, Kikkawa DO, Korn BS. (2021) Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit:1–4. https://doi.org/10.1080/01676830.2021.1903044
Khatri A, Chang K-M, Berlinrut I, Wallach F (2021) Mucormycosis after coronavirus disease 2019 infection in a heart transplant recipient - case report and review of literature. J Mycol Med 31:101125. https://doi.org/10.1016/j.mycmed.2021.101125
Maini A, Tomar G, Khanna D, Kini Y, Mehta H, Bhagyasree V (2021) Sino-orbital mucormycosis in a COVID-19 patient: a case report. Int J Surg Case Rep 82:105957. https://doi.org/10.1016/j.ijscr.2021.105957
Ahmadikia K, Hashemi SJ, Khodavaisy S, Getso MI, Alijani N, Badali H, et al. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses 2021; published online Feb 16. https://doi.org/10.1111/myc.13256
Tabarsi P, Khalili N, Pourabdollah M, Sharifynia S, Safavi Naeini A, Ghorbani J, et al. COVID-19 associated rhinosinusitis mucormycosis due to Rhizopus oryzae: a rare but potentially fatal infection occurring after treatment with corticosteroids. Res Square 2021. https://doi.org/10.21203/rs.3.rs-398594/v2
Kanwar A, Jordan A, Olewiler S, Wehberg K, Cortes M, Jackson BR (2021) A fatal case of Rhizopus azygosporus pneumonia following COVID-19. J Fungi 7:174. https://doi.org/10.3390/jof7030174
Waizel-Haiat S, Guerrero-Paz JA, Sanchez-Hurtado L, Calleja-Alarcon S, Romero-Gutierrez L (2021) A case of fatal rhino-orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID-19. Cureus 13:e13163. https://doi.org/10.7759/cureus.13163
Arana C, Cuevas Ramírez RE, Xipell M, Casals J, Moreno A, Herrera S, et al. (2021) Mucormycosis associated with covid-19 in two kidney transplant patients. Transpl Infect Dis: e13652. https://doi.org/10.1111/tid.13652
John TM, Jacob CN, Kontoyiannis DP (2021) When uncontrolled diabetes mellitus and severe COVID-19 converge: the perfect storm for mucormycosis. J Fungi 7:298. https://doi.org/10.3390/jof7040298
Al-Hatmi AMS, Mohsin J, Al-Huraizi A, Khamis F (2021) COVID-19 associated invasive candidiasis. J Infect 82:e45–e46. https://doi.org/10.1016/j.jinf.2020.08.005
Silva DL, Lima CM, Magalhães VC, Baltazar LM, Peres NT, Caligiorne R et al (2021) Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J Hosp Infect 2021(113):145–154. https://doi.org/10.1016/j.jhin.2021.04.001
Posteraro B, Torelli R, Vella A, Leone PM, De Angelis G, De Carolis E et al (2020) Pan-echinocandin-resistant Candida glabrata bloodstream infection complicating COVID-19: a fatal case report. J Fungi 6:163. https://doi.org/10.3390/jof6030163
Onac I, Ali S, Mahto A, Rutherford A, Galloway J, Nagra D (2020) EP13 A case of Candida albicans septic sacroiliitis complicating severe COVID-19 infection. Rheumatol Adv Pract 4:23. https://doi.org/10.1093/rap/rkaa052.012
Allaw F, Kara Zahreddine N, Ibrahim A, Tannous J, Taleb H, Bizri AR et al (2021) First Candida auris outbreak during a COVID-19 pandemic in a tertiary-care center in Lebanon. Pathog 10:157. https://doi.org/10.3390/pathogens10020157
de Almeida JN, Francisco EC, Hagen F, Brandão IB, Pereira FM, Presta Dias PH et al (2021) Emergence of Candida auris in Brazil in a COVID-19 intensive care unit. J Fungi 7:220. https://doi.org/10.3390/jof7030220
Prestel C, Anderson E, Forsberg K, Lyman M, de Perio MA, Kuhar D et al (2021) Candida auris outbreak in a COVID-19 specialty care unit - Florida, July-August 2020. MMWR Morb Mortal Wkly Rep 70:56–57
Villanueva-Lozano H, Treviño-Rangel R de J, González GM, Ramírez-Elizondo MT, Lara-Medrano R, Aleman-Bocanegra MC et al (2021) Outbreak of Candida auris infection in a COVID-19 hospital in Mexico. Clin Microbiol Infect 27:813–16. https://doi.org/10.1016/j.cmi.2020.12.030
Rodriguez JY, Le Pape P, Lopez O, Esquea K, Labiosa AL, Alvarez-Moreno C. Candida auris: a latent threat to critically ill patients with COVID-19. Clin Infect Dis 2020; published online Oct 18. https://doi.org/10.1093/cid/ciaa1595
Arastehfar A, Shaban T, Zarrinfar H, Roudbary M, Ghazanfari M, Hedayati M-T et al (2021) Candidemia among Iranian patients with severe COVID-19 admitted to ICUs. J Fungi 7:280. https://doi.org/10.3390/jof7040280
de Almeida JN, Moreno J, Francisco L, Noronha Marques EC, Mendes G, Barberino AV et al (2021) Trichosporon asahii superinfections in critically ill COVID-19 patients overexposed to antimicrobials and corticosteroids. Mycoses. https://doi.org/10.1111/myc.13333
Passerini M, Terzi R, Piscaglia M, Passerini S, Piconi S (2020) Disseminated cryptococcosis in a patient with metastatic prostate cancer who died in the coronavirus disease 2019 (COVID-19) outbreak. Cureus 12:e8254. https://doi.org/10.7759/cureus.8254
Cafardi J, Haas D, Lamarre T, Feinberg J. (2021) Opportunistic fungal infection associated with COVID-19. Open Forum Infect Dis: ofab016. https://doi.org/10.1093/ofid/ofab016
Ventoulis I, Sarmourli T, Amoiridou P, Mantzana P, Exindari M, Gioula G et al (2020) Bloodstream infection by Saccharomyces cerevisiae in two COVID-19 patients after receiving supplementation of Saccharomyces in the ICU. J Fungi 6:98. https://doi.org/10.3390/jof6030098
Chang CC, Senining R, Kim J, Goyal R (2020) An acute pulmonary coccidioidomycosis coinfection in a patient presenting with multifocal pneumonia with COVID-19. J Investig Med high impact case reports 8:2324709620972244. https://doi.org/10.1177/2324709620972244
Shah AS, Heidari A, Civelli VF, Sharma R, Clark CS, Munoz AD et al (2020) The coincidence of 2 epidemics, coccidioidomycosis and SARS-CoV-2: a case report. J Investig Med high impact case reports 8:2324709620930540. https://doi.org/10.1177/2324709620930540
de Macedo PM, Freitas DFS, Varon AG, da Cruz LC, Fonseca Ferreira LC, d’Avila Freitas A et al (2020) COVID-19 and acute juvenile paracoccidioidomycosis coinfection. PLoS Negl Trop Dis 14:e0008559. https://doi.org/10.1371/journal.pntd.0008559
Bertolini M, Mutti MF, Barletta JA, Falak A, Cuatz D, Sisto A et al (2020) COVID-19 associated with AIDS-related disseminated histoplasmosis: a case report. Int J STD AIDS 31:1222–1224. https://doi.org/10.1177/0956462420957518
Basso RP, Poester VR, Benelli JL, Stevens DA, Zogbi HE, da S Vasconcellos IC, et al. COVID-19-associated histoplasmosis in an AIDS Patient. Mycopathologia 2021;186:109–12. https://doi.org/10.1007/s11046-020-00505-1
Messina FA, Marin E, Caceres DH, Romero M, Depardo R, Priarone MM et al (2020) Coronavirus disease 2019 (COVID-19) in a patient with disseminated histoplasmosis and HIV-a case report from Argentina and literature review. J Fungi 6:275. https://doi.org/10.3390/jof6040275
Mang S, Kaddu-Mulindwa D, Metz C, Becker A, Seiler F, Smola S et al (2021) Pneumocystis jirovecii pneumonia and severe acute respiratory syndrome coronavirus 2 coinfection in a patient with newly diagnosed HIV-1 infection. Clin Infect Dis 72:1487–1489. https://doi.org/10.1093/cid/ciaa906
Menon AA, Berg DD, Brea EJ et al (2020) A case of COVID-19 and Pneumocystis jirovecii coinfection. Am J Respir Crit Care Med 202:136–138. https://doi.org/10.1164/rccm.202003-0766LE
Poignon C, Blaize M, Vezinet C, Lampros A, Monsel A, Fekkar A (2020) Invasive pulmonary fusariosis in an immunocompetent critically ill patient with severe COVID-19. Clin Microbiol Infect 26:1582–1584. https://doi.org/10.1016/j.cmi.2020.06.026
Khan N, Gutierrez CG, Martinez DV, Proud KC (2020) A case report of COVID-19 associated pulmonary mucormycosis. Arch Clin Cases 07:46–51. https://doi.org/10.22551/2020.28.0703.10172
Johnson AK, Ghazarian Z, Cendrowski KD, Persichino JG (2021) Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med Mycol Case Rep 32:64–67. https://doi.org/10.1016/j.mmcr.2021.03.006
Bellanger A-P, Navellou J-C, Lepiller Q, et al. Mixed mold infection with Aspergillus fumigatus and Rhizopus microsporus in a severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) patient. Infect Dis now 2021; published online Jan 27. https://doi.org/10.1016/j.idnow.2021.01.010
Abdalla S, Almaslamani MA, Hashim SM, Ibrahim AS, Omrani AS (2020) Fatal coronavirus disease 2019-associated pulmonary aspergillosis; a report of two cases and review of the literature. ID Cases 22:e00935. https://doi.org/10.1016/j.idcr.2020.e00935
Yin CH, Wang C, Tang Z, Zhang SW, Wang BS (2004) Clinical analysis of 146 patients with critical severe acute respiratory syndrome in Beijing areas. Clin J Emerg Med 1:12–14
Thevissen K, Jacobs C, Holtappels M, Toda M, Verweij P, Wauters J (2020) International survey on influenza-associated pulmonary aspergillosis (IAPA) in intensive care units: responses suggest low awareness and potential underdiagnosis outside Europe. Crit Care 24:84. https://doi.org/10.1186/s13054-020-2808-8
Jenks JD, Hoenigl M (2018) Treatment of aspergillosis J Fungi 4:98. https://doi.org/10.3390/jof4030098
Fekkar A, Poignon C, Blaize M, Lampros A (2020) Fungal infection during COVID-19: Does Aspergillus mean secondary invasive aspergillosis? Am J Respir Crit Care Med 202:902–903. https://doi.org/10.1164/rccm.202005-1945LE
de León-Borrás R, DelPilar-Morales E, Rivera-Pérez N, Pallens-Feliciano M, Tirado-Gómez M, González-Sepúlveda L, et al. Factors associated to invasive fungal infection in hispanic patients with hematological malignancies. Bol Asoc Med P R 2017;109:43–8
Jolobe OM (2020) Air leaks, pneumatoceles, and air spaces in COVID-19 pneumonia. Am J Emerg Med 2020:30792. https://doi.org/10.1016/j.ajem.2020.08.098
Issara-Amphorn J, Surawut S, Worasilchai N, Thim-uam A, Finkelman M, Chindamporn A et al (2018) The synergy of endotoxin and (1→3)-β-D-glucan, from gut translocation, worsens sepsis severity in a lupus model of Fc gamma receptor IIb-deficient mice. J Innate Immun 10:189–201. https://doi.org/10.1159/000486321
Kullberg BJ, Arendrup MC (2015) Invasive candidiasis. N Engl J Med 373:1445–1456. https://doi.org/10.1056/NEJMra1315399
Marra AR, Camargo LFA, Pignatari ACC, Sukiennik T, Petersen Behar PR, Servolo Medeiros EA et al (2011) Nosocomial bloodstream infections in Brazilian hospitals: analysis of 2,563 cases from a prospective nationwide surveillance study. J Clin Microbiol 49:1866–1871. https://doi.org/10.1128/JCM.00376-11
Centers for Disease Control and Prevention. https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html [Accessed 24 June 2021]
Gasparoto TH, de Oliveira CE, Vieira NA, Porto VC, Gasparoto CT, Campanelli AP et al (2012) The pattern recognition receptors expressed on neutrophils and the associated cytokine profile from different aged patients with Candida-related denture stomatitis. Exp Gerontol 47:741–748. https://doi.org/10.1016/j.exger.2012.07.003
Marteau PR, de Vrese M, Cellier CJ, Schrezenmeir J (2001) Protection from gastrointestinal diseases with the use of probiotics. Am J Clin Nutr 73:430S-436S. https://doi.org/10.1093/ajcn/73.2.430s
Erener S (2020) Diabetes, infection risk and COVID-19. Mol Metab 39:101044. https://doi.org/10.1016/j.molmet.2020.101044
Petrikkos G, Tsioutis C (2018) Recent advances in the pathogenesis of mucormycoses. Clin Ther 40:894–902. https://doi.org/10.1016/j.clinthera.2018.03.009
Morales-Franco B, Nava-Villalba M, Medina-Guerrero EO, Sánchez-Nuño YA, Davila-Villa P, Anaya-Ambriz EJ, et al. Host-pathogen molecular factors contribute to the pathogenesis of Rhizopus spp. in diabetes mellitus. Curr Trop Med reports 2021;8:6–17. https://doi.org/10.1007/s40475-020-00222-1
Thomas CF, Limper AH (2004) Pneumocystis pneumonia. N Engl J Med 350:2487–2498. https://doi.org/10.1056/NEJMra032588
Vizcarra P, Pérez-Elías MJ, Quereda C, Moreno A, Vivancos MJ, Dronda F et al (2020) Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV 7:e554–e564. https://doi.org/10.1016/S2352-3018(20)30164-8
Xu Z, Zhang C, Wang F-S (2020) COVID-19 in people with HIV. Lancet HIV 7:e524–e526. https://doi.org/10.1016/S2352-3018(20)30163-6
Acknowledgements
R.G.V. and S.M.R. are grateful to Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación of Argentina (PICT-2018-03781). The work of S.S. was supported by the Intramural Research Program of the National Institutes of Health, Clinical Center, Department of Laboratory Medicine.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Vitale RG, Romero SM, Giudicessi SL, Seyedmousavi S, and Afeltra J. The first draft of the manuscript was written by Vitale RG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable for this section.
Consent to participate
Not applicable for this section.
Consent for publication
Not applicable for this section.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Carlos Pelleschi Taborda
Rights and permissions
About this article
Cite this article
Vitale, R.G., Afeltra, J., Seyedmousavi, S. et al. An overview of COVID-19 related to fungal infections: what do we know after the first year of pandemic?. Braz J Microbiol 53, 759–775 (2022). https://doi.org/10.1007/s42770-022-00704-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00704-6