Abstract
This work evaluated the agronomic and soil fertility effects of using municipal wastewater or anaerobically treated wastewater for irrigation and applying biochar to a soil from the Guinea savanna agroecological zone of Ghana. For this purpose, untreated municipal wastewater (WW), the effluent of an anaerobic wastewater filtration system (TWW), and clean water (CW) were used as irrigation water in a pot trial. Additionally, rice-husk biochar in the form of raw biochar (RB), water-washed biochar (WB), and biochar used as wastewater filter material (FB) were added to the soil, testing the influence on soil fertility and crop yield. Lettuce and carrot were selected for the pot study, grown on soil mixed with the biochar types at 20 t ha−1 and irrigated with either WW, TWW, or CW. Our results indicated higher crop growth morphology and yields (up to 90% increase) by WW and TWW than CW. The average yield of carrot (34.1 g pot−1) and lettuce (29.3 g pot−1) with TWW irrigation were the highest, followed by 31.2 and 27 g pot−1 with WW, then the lowest yields of 21.7 and 19.5 g pot−1 of carrot and lettuce irrigated with CW respectively. Compared to WW, TWW was more beneficial to plant development, causing an up to 10% increase in crop yields. Soils with FB and WB produced similar agronomic effects and plant nutrient concentrations but were lower than pots amended with RB. Nevertheless, combining RB with TWW showed increasing effects on pH, CEC, and P availability in the highly weathered acidic soil. The results suggest a beneficial effect of biochar-filtered wastewater on soil fertility and crop growth, offering the potential to enhance resource use efficiency in irrigated urban agroecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Semi-arid regions, such as Sub-Saharan Africa, face challenges regarding freshwater availability for irrigation during the dry seasons, making wastewater an option for urban horticulture (Helmecke et al. 2020). However, wastewater poses the risk of pathogen and chemical contamination, especially in developing countries where most wastewater is used untreated (Gallego-Schmid and Tarpani 2019). Over the years, several technologies have been developed to treat wastewater for agricultural purposes, but these options are sophisticated and generally expensive, thus being unavailable to poorly resourced farmers (Gallego-Valero et al. 2021). Kaetzl et al. (2020) reiterated the need to localize the implementation of affordable on-farm wastewater treatment systems for safer urban crop production to ensure food security and environmental quality. Besides being an alternative to freshwater, wastewater primarily contains plant-available nutrients and becomes a reliable source of nitrogen and phosphorous for plant growth (Helmecke et al. 2020). Following wastewater irrigation in semi-arid areas, Akoto-Danso et al. (2019) reported up to 20-fold increases in crop yield and improved soil properties compared to freshwater irrigation.
Biochar is simply a pyrolyzed organic material mostly produced from agricultural biomass (Lehmann and Joseph 2015). The addition of biochar has been studied extensively and proven to enhance the chemical, physical, and biological properties of poor soils (Lehmann et al. 2011; Mitchell et al. 2015). Increased crop yield is reported in biochar-amended soils by improving organic carbon, nutrient and water holding capacity, pH buffering capacity, and biological indicators (Häring et al. 2017; Xu et al. 2012). In acidic tropical soils, biochar increased the yield of amaranth (Manka’abusi et al. 2019), maize (Frimpong et al. 2021), and cowpea (Yeboah et al. 2020) compared to the unamended control.
In the last decade, biochar from residual biomass also gained importance as a low-cost alternative to activated carbon for wastewater treatment (Kaetzl et al. 2020). Kaetzl et al. (2019a) reported an efficient removal capacity of chemical oxygen demand (COD) by Miscanthus biochar in a laboratory experiment comparing biochar’s performance to sand as a filter medium. In an extended field study using rice-husk biochar as a filter medium, the concentration of plant-essential phosphorus and nitrogen in the filtered wastewater was maintained (Kaetzl et al. 2019b). Biochar has also been proven effective for heavy metal reduction in wastewater by several studies (Fan et al. 2019). Nutrient loading on biochar surfaces during the filtration process is proposed by several studies (Foereid 2015; Mizuta et al. 2004; Pathy et al. 2021). However, Enaime et al. (2020) stated the potential leaching of dissolved mineral compounds from the biochar into the filtered wastewater. Agronomically, biochar-filtered wastewater was superior to crop yield compared to the untreated counterpart (Kaetzl et al. 2020). Also, in a pot trial by Werner et al. (2018) testing the agronomic benefits of filterchar (biochar after filtration), both untreated biochar and filterchar similarly improved aboveground biomass compared to unamended control.
Several studies support the adoption of biochar in wastewater treatment technology (Dutta et al. 2021; Enaime et al. 2020; Kaetzl et al. 2018), yet there is still little information regarding the agronomic importance of biochar in wastewater treatment. Hence, this study aimed to test the following hypotheses: (i) Biochar-filtered wastewater has more beneficial effects on crop performance than untreated wastewater because biochar releases water-soluble nutrients, (ii) the nutrient depletion of the filterchar renders it less suitable for soil amendment than untreated biochar. This study filtered wastewater through a rice husk biochar medium in a simple on-farm water treatment system. The yield and nutrient concentrations of carrot and lettuce were tested under filtered and untreated wastewater irrigation on biochar-amended acidic soil.
2 Materials and Methods
2.1 Study Area
The study employed a pot study carried out in a greenhouse in Tamale, the northern region of Ghana (9°28′28.75″ N, 0°50′53.48″ W) (Fig. 1). The site is in the Guinea savanna zone, characterized by semi-arid climatic conditions. The rainfall pattern is monomodal, which occurs between April and October. The daily mean temperature is 28.9 °C, and the annual mean precipitation is 1090 mm.
Map showing agroecological zones of Ghana (Germer and Sauerborn, 2008). The experimental site located in Tamale, the northern region of Ghana (9°28′28.75″ N, 0°50′53.48″ W) is indicated with a star
2.2 Biochar Production and Irrigation Water Treatment
The biochar was locally produced from an abundant rice husk biomass using a modified ELSA gasifier designed from an empty oil barrel (Steiner et al. 2018). This untreated rice-husk biochar is termed raw biochar (RB) in this study. For wastewater purification, a low-cost and simple on-farm wastewater treatment system described by Kaetzl et al. (2018) was used for wastewater filtration. Briefly, the system was operated as two-stage filtration steps under anaerobic conditions by utilizing rice husk biochar as the filter medium. With a hydraulic loading rate of 0.05 mh−1, the filter removed up to 3 log10-units of the fecal indicator bacteria, 89% of the COD, and turbidity up to 92% (Kaetzl et al. 2019b).
The biochar filter material (filterchar) was retrieved after 240 days and sun-dried to a constant weight. The dried biochar filter was then applied as an amendment material and referred to in this study as filterchar (FB). Water-washed biochar (WB) was obtained by subjecting the raw biochar to repeated washing with deionized water. A ratio of 1:5 biochar and deionized water was shaken at 200 rpm for 12 h. The content was decanted, and the process was repeated until the electrical conductivity (EC) was reduced by about 95%. The washed biochar was sieved and dried at 60 °C until a constant weight was reached. Ash content and total nutrient concentration of RB, FB, and WB were determined (Table S1). The particle size of all the rice husk biochar types was below 2 mm and much smaller in the FB due to the pre- and post-filtration handling. The ash contents were 42.98%, 33.24%, and 30.05% in RB, WB, and FC, respectively.
2.3 X-ray Diffraction and Brunauer Emmett-Teller Analysis of Biochar
The X-ray diffraction (XRD) measurements of biochar were done using a D8 ADVANCE (Bruker) powder diffractometer with theta-theta geometry, Cu Kα radiation (λ = 0.15406 nm, 40 kV, 40 mA), and an energy-dispersive detector (LynxExe-1D). Angles from 10 to 80° 2Î were measured with a step size of 0.015° and a time per step of 1.5 s. All measurements were conducted at room temperature and atmospheric pressure. Brunauer-Emmett-Teller (BET) specific surface area and Barrett-Joyner-Halenda (BJH) pore size and volume of the biochar samples were evaluated using Autosorb 2000 analyzer (Quantachrome NovaWin10). The samples were outgassed for 3 h at 130 C for pretreatment. The N2-adsorption isotherms of the biochar were determined at 77 K with pressures (P/P0) up to 0.995 and BET at a relative pressure P/P0 = 0.05–0.30.
2.4 Experimental Setup and Agronomic Practice
Petroplinthic Cambisol soil (IUSS Working Group WRB 2014) to a depth of 20 cm from the Guinea savanna of northern Ghana was used to conduct the pot experiment in a greenhouse. Initially, the soil was classified as sandy loam with 45.7% sand, 47% silt, and clay content of 5.9%. The soil contained organic carbon (SOC) concentration of 4.1 g kg−1, a total N content of 0.4 g kg−1, and a cation exchange capacity (CEC) of 33 mmolc kg−1 (Häring et al. 2017). The potting soil contained 20 t ha−1 of either RB, WB, or FB, thoroughly mixed and transferred to 6-L plastic containers. The biochar application rate was set in reference to previous studies by Häring et al. (2017) and Akoto-Danso et al. (2019). Treatments were triplicated and laid in a completely randomized block design. The carrot was directly planted by placing five seeds in each pot. Thinning-out was performed after germination, allowing a plant per pot to grow for 8 weeks. A 14-day lettuce nursery was transferred and grown for four weeks in a similar experimental setup as the carrot. Without further soil amendment, the lettuce cycle was repeated for another 4 weeks on the same treatment pot to study the treatment effect on the second lettuce growth cycle and to compensate for the 8-week duration of the single carrot cycle. The potting soil was relatively low in nutrients, pH, and organic matter. Hence, each treatment pot received background chemical fertilization of NPK (nitrogen, phosphorus and potassium) 15:15:15 at 102 kg ha−1 rate, including the control pots. The background fertilization was done to mimic regular agricultural practice in tropical savanna soils at a reduced rate, as farmers do not grow without fertilizer. Both lettuce and carrot crops received a 200 ml daily dose of either WW, TWW, or CW.
During the second and fourth week after germination, a single-photon avalanche diode (SPAD; 502, Konica Minolta, Tokyo, Japan) was used to measure the chlorophyll content. The carrot root’s volume, weight, height, and core diameter were measured at harvest. Additionally, leaf height and head diameter were measured on the aboveground biomass of a carrot and lettuce. For further plant nutrient analysis, the lettuce, root, and leaf biomass of the carrot were oven-dried separately at 60 °C to a constant weight. Similarly, soil samples from all pots were air-dried, packaged, and transported to Germany for further analysis
2.5 Water Analysis
Irrigation water types were sampled twice every week and immediately measured for NO3−-N (Cataldo et al. 1975), NH4+-N (Koroleff 1983), and PO4−-P (Ohno and Zibilske 1991). Light absorption at the specific wavelengths was measured with a UV/VIS spectrophotometer (Pharo 300 Spectroquant, Merck GmbH, Darmstadt, Germany). Electrical conductivity and pH were determined with a standard calibrated EC meter (Basic 20, Crison Instruments S.A., Spain) and pH meter (Basic 20, Crison Instruments S.A., Spain) respectively. Portions of the water were acid-treated and transported to Germany for elemental analysis using an ICP-OES (Ciros CCD, SPECTRO Analytical Instruments GmbH, Kleve, Germany). Average plant essential nutrients such as PO4−-P, NO3−-N, NH4+-N and TOC were 3.73 mg L−1, 0.88 mg L−1, 12.97 mg L−1, and 6.34 mg L−1 in WW and 2.95 mg L−1, 0.82 mg L−1, 12.23 mg L−1, and 5.01 mg L−1 in TWW, respectively (Table S2).
2.6 Analysis of Soil and Plant Nutrient Concentration
The total nutrient concentration (Fe, Ca, Mg, K, and P) in soil and plant were analyzed with ICP-OES (Ciros CCD, SPECTRO Analytical Instruments GmbH, Kleve, Germany) after microwave digestion with concentrated nitric acid. Dry combustion in an elemental analyzer (Vario max cube, Elementar Analysesysteme GmbH, Hanau, Germany) was used to determine total carbon and nitrogen concentrations. The pH of the soil was determined in CaCl2 solution (1:2.5 w/v) and electrical conductivity by (1:5 w/v) deionized water. The available P extraction method described by Bray and Kurtz (1945), with an acid fluoride extractant in a 1:7 soil: solution ratio (w: vol) was used for available P in the soil. Orthophosphates in the solutions were determined by using the molybdenum blue method (Murphy and Riley 1962). Exchangeable ions (Ca2+, Mg2+, K+, Na+, and Al3+) in soil were extracted with NH4Cl by adding 10 ml NH4Cl to 2.5 g soil and allowed to stay for 24 h. The content was filtered with Whatman paper and further percolated with NH4Cl for 4 h to a volume of 100 ml before measurement with ICP-OES Ciros CCD, SPECTRO Analytical Instruments GmbH, Kleve, Germany).
2.7 Data Analysis
Significant differences of means were assessed using multifactorial analysis of variance (MANOVA, p < 0.05), considering water quality and biochar as factors. Data from carrot and lettuce cultivated setup were managed separately. A log transformation was applied to CEC, plant Ca, and K concentration after the Shapiro-Wilk test was performed to check the normal distribution of the dataset. Turkey’s HSD post hoc test was used to identify the significance level of treatments at p < 0.05. Pearson moment correlation was additionally performed between plant tissue concentration and yield. The statistical analyses were carried out using R Language and Environment for Statistical Computing (R Core Team 2017) and figure created with OriginPro 2021 software (Origin Lab Corporation, Northampton, USA).
3 Results
3.1 Growth Indicators and Biomass Production
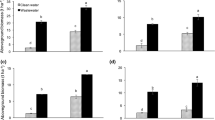
Plant morphological parameters, including root dimensions of carrot (Table S4), root:shoot ratio, and chlorophyll concentration (Table S6), showed a significant (p < 0.05) improvement with wastewater (treated and untreated) irrigation. This resulted in increased biomass production of carrot (54%), first lettuce (33%), and second lettuce (62%) relative to tap water. Comparatively, biochar-filtered wastewater was about 10% more beneficial to plant productivity than untreated wastewater (Figs. 2 and 3). The root:shoot ratio in carrot was lowered equivalently by TWW and WW compared to CW but remained unchanged in both lettuce growth cycles. The irrigation effect on leaf chlorophyll concentrations was significant (p < 0.05) with a mean of 19.9 and 35.7 SPAD units in carrot and lettuce respectively (Table S3). At an application rate of 20 t ha−1, rice husk biochar irrespective of the quality (RB, FB, and WB) significantly increased the lettuce yield in the first and second cultivation periods (Fig. 3). In contrast to the lettuce crop, there was no biochar effect on the growth indicators and total biomass production of carrot compared to the unamended control (Fig. 3). Background fertilization with NPK at the start of the experiment enhanced the growth of first lettuce producing total biomass similar to the second lettuce that received prolonged wastewater irrigation (Fig. 2).
Total fresh biomass of carrot after harvest. Upper and lower columns represent above- and below-ground biomass, respectively. Clean water (CW), treated wastewater (TWW) and untreated wastewater (WW) were applied as irrigation water and raw biochar (RB), filterchar (FB) and washed biochar (WB) as soil amendments. Letters indicate a significant difference of the means (MANOVA, p < 0.05) after post hoc analysis. Error bars denote the standard deviation of the mean (n =3)
Total fresh biomass of first lettuce (A) and second lettuce (B) per pot. The upper and lower columns represent above- and below-ground biomass, respectively. Clean water (CW), treated wastewater (TWW) and untreated wastewater (WW) were applied as irrigation water and raw biochar (RB), filterchar (FB) and washed biochar (WB) as soil amendments. Letters indicate a significant difference of the means (MANOVA, p < 0.05) after post hoc analysis. Error bars denote the standard deviation of the mean (n =3)
3.2 Taproot and Leaf Nutrient Concentration of Carrot
At the matured stage, the leaf and root parts of the carrot were analyzed separately for nutrient concentration. Amongst the plant’s macronutrients and Fe, the concentration of Ca and K constituted about 90% of the total nutrient in the carrot tissues. Except for P, which was more concentrated in the root, all other nutrients were several folds higher in the leaf relative to the taproot. For instance, Ca, Mg, and Fe were consecutively 8-, 3- and 2-fold more concentrated in the leaf than in the taproot (Fig. 4). Irrigation with TWW did not alter the amount of N, P, and K in the carrot tissue but reduced Fe intake by 13% compared to WW. Within the various biochar types, untreated biochar (21.7 g kg−1 K) had a pronounced impact on total K concentration in carrots compared to filterchar (18.8 g kg−1 K) and water-washed biochar (19.7 g kg−1 K). Although not significant, untreated biochar (RB) lead to slightly higher contents of all other nutrients of carrot tissues than the filter and water-washed biochar (Fig. 4).
Average concentration of nitrogen (A), phosphorus (B), potassium (C), calcium (D) in carrot leaf (upper columns) and root (lower columns) tissue produced in biochar amended and irrigated soil. Clean water (CW), treated wastewater (TWW) and untreated wastewater (WW) were applied as irrigation water and raw biochar (RB), filterchar (FB) and washed biochar (WB) as soil amendments. Letters indicate a significant difference of the means (MANOVA, p < 0.05) after post hoc analysis. Error bars denote the standard error of the mean (n = 3)
3.3 Foliar Nutrient Concentration of Lettuce
In lettuce biomass, biochar and irrigation treatment had varying effects on nutrient concentrations in the first and second cropping periods. Specifically, the first lettuce Fe in the control soil was 0.59 g kg−1 compared to 0.70 g kg−1 in the second cropping period. However, Ca concentration was reduced by up to 40% in the second cropping relative to lettuce from the first cropping season. In a similar pattern, WW and TWW irrigation during the first cropping significantly improved the amount of K (35%), N (14%), and Mg (7%), while a percentage increase for K, P, N, and Mg was less than 10% in the second season. Biochar’s impact on lettuce nutrient composition was only significant in the case of K and Mg for both cropping seasons, even though the other measured nutrient were slightly increased.
3.4 Treatment and Plant Effect on Cation Exchange Capacity of Soil
The ability of soil to retain cations (CEC) was determined for both lettuce and carrot soils after 8 weeks of biochar application and wastewater irrigation. The various exchangeable cations (Ca2+, Mg2+, K+, and Na+) in lettuce- (48.50 mmolc kg−1) cultivated soil were slightly lower than soil used for carrot (46.26 mmolc kg−1) cultivation. The reduction was noticeable in K+ (1.62 mmolc kg−1) and Na+ (4.55 mmolc kg−1) compared to K+ (2.67 mmolc kg−1) and Na+ (3.44 mmolc kg−1) in lettuce-cultivated soil. The pattern of irrigation effect on total CEC was similar for both treated and untreated wastewater, which were significantly higher than the clean water irrigated soil (Fig. 6). Amending the soil with biochar showed a substantial increase in K+ (45%) and Na+ (15%), contributing to a significant increase in total CEC compared to the unamended soil. The dominant cation was Ca2+, constituting about 80% of the total CEC (Fig. 6).
3.5 Soil Chemical Parameters
After the cropping period, the soil’s pH, total N, P, K, and plant-available P were significantly (p < 0.05) improved following irrigation with wastewater (Table 1). Irrigation with TW and WW had a similar effect on soil chemical parameters. The SOC was increased by 9% in the various wastewater-irrigated soils but was not significantly different to the clean water treatment. Biochar addition increased SOC (41%), pH (8%), Ntot (11%), Ktot (25%), Ptot (5%), and Pavail (20%) compared to the unamended control (Table 1). A statistically significant (p < 0.05) increase was however indicated only in SOC, Ktot, and pH (Table 1). Biochar effect on soil nutrient concentration was reduced in WB and FB compared to the RB-amended soils. The reduction was apparent in the soil’s Ktot and Pavail concentration by 10% and 12%, respectively. Lettuce and carrot cultivation caused an alternating effect on K and P in the soil. The amount of K increased while P was reduced in lettuce cultivated soil, corresponding with the total nutrient concentration in their respective biomass.
4 Discussion
The concentration of essential plant nutrients in domestic wastewater is reported in many folds and is directly transferred to the soil (Adrover et al. 2012; Akoto-Danso et al. 2019; Enaime et al. 2020; Kaetzl et al. 2018). These nutrients contributed to an increase in total N (+20%), P (+17%), K (+18%), and 15% available P concentrations of WW compared to CW-irrigated soil. As earlier reported by Kaetzl et al. (2020), plant essential nutrients were retained in TWW after the filtration process, leading to enhanced macronutrients in WW and TW-irrigated soil compared to the soils with CW irrigation. Domestic wastewater comprises 1% dissolved organic matter that alters soil carbon stock following long-term irrigation. These organic compounds might have facilitated the increased 10% SOC concentration in soils irrigated with WW than CW, confirming a previous study by Asirifi et al. (2021b) with similar SOC inputs in soil irrigated with wastewater. The observed equivalent SOC in treated and untreated wastewater irrigated soil could be linked to the biochar used as a filter material. As the treatment removed significant amounts of suspended solids from the treated wastewater, leached dissolved organic components from the biochar and hydrolyzed organic substances under anaerobic conditions could have substituted the removed particulate organic matter (Kaetzl et al. 2020). The CEC moved from 40 mmolc kg−1 in CW irrigated soil to 48 mmolc kg−1 in WW and TWW. Wastewater irrigation slightly enhanced the soil organic matter, creating more available sites for cation adsorption on the soil colloids’ surface (Ramos et al. 2018). High sodium ions in wastewater might have directly increased the CEC in WW-irrigated soil. The exchangeable sodium percentage of WW irrigated soil was 8%, five-folds higher than CW irrigated soil, presumably resulting from household activities that utilize wide varieties of detergent. However, the soil CEC constituted over 85% divalent ions of Ca2+ and Mg2+ (Fig. 6), which has a high affinity to the surface of soil colloids and therefore displace the monovalent sodium (Yan and Hou 2018). This is indicated by the low electrical conductivity of the soil, suggesting a low potential risk of salination by Na+ even in a long period of wastewater irrigation (Hadi and Karimi 2012).
Fundamentally, biochar is a stable carbon material and may differ in functions depending on many properties, including biomass type, application rate, and soil quality. In the tropical savanna soil with low organic matter, the addition of 20 t ha−1 rice husk biochar directly increased the soil carbon in RB- (35%), WB- (25%), and FB- (27%) amended soil. The loss of DOC and inorganic C components of biochar through either the washing or the water filtration process may have caused the subsequent reduction of SOC in WB- and FB-amended soil compared to soil with untreated biochar. In a similar study involving rice husk biochar, Smebye et al. (2016) reported reduced SOC in water-washed biochar-amended soil. The RB contained 43% ash and correlated significantly with pH, CEC, and total K improvement in the poor acidic soil. The high-ashed biochar provided calcium carbonate and potassium to the soil, allowing more binding surfaces to hold cations. In agreement with this result, Asirifi et al. (2022) and Häring et al. (2017) reported an improved pH and soil CEC in a degraded acidic Cambisol and Ferrosol following biochar amendment. Häring et al. (2017) emphasized a short-lived effect of biochar on soil pH and CEC due to leaching, highlighting the importance of biochar bond nutrients and ash components in degraded soil.
Similarly, reducing the ash content of biochar to 28% (WB) and 25% (FB) through washing or filtration corresponded to lowering pH, K, and CEC in the soil relative to the unamended control. Werner et al. (2018) also found a reduction in biochar-bound nutrients like P, Mg, and K after wastewater percolation that decreased lettuce biomass compared to untreated biochar. The doubled surface area of FB (102.78 m2 g−1) compared to RB (66.67 m2 g−1) resulted mainly from the removal of soluble components like ash and the physical handling of FB during the filtration process. The ash content was reduced from 43 to 30% in the FB. In addition, the production process (loading the barrel, filtration, and drying) crushed the FB to a much smaller size of 0.125–0.250 mm (68%) compared to the RB, which had about 30% of the particles below 0.250 mm. The improved surface area did not influence nutrient retention and therefore on crop yield as the study was conducted in a pot without nutrient losses through leaching.
The crop types (carrot and lettuce) had varying effects on the soil’s P and K contents, indicating the importance of plant factors on the functions of amendments in the soil. For instance, the reduced P content in lettuce-cultivated soil corresponded with the high P uptake by the lettuce crop, probably due to its root density that might have interacted with P in the soil. Such effects were observed by Föhse et al. (1991) and Bhattacharya (2018), where root hair stimulated up to 90% of P uptake in soil with low extractable P. The K requirement for carrots is high as it plays a critical role in water and nutrient transport within the tissue (Chaudhary et al. 2020). This increased demand for potassium explains the depletion in carrot-cultivated soil and correlates with the K concentration in the carrot tissues. This confirms the previous study of Shikha et al. (2016) and El-Tohamy et al. (2011) that reported a strong correlation between potassium availability and carrot biomass production.
The enhanced growth indicator parameters (root and leaf dimensions) and total biomass production are explained by direct nutrient addition through wastewater irrigation and its possible influence on the soil’s microbial activities (Asirifi et al. 2021b). A significant increase in crop yield is mostly guaranteed under wastewater irrigation due to its fertigation potential (Adrover et al. 2012). As indicated in lettuce production, the wastewater effect on yield increased with time (Fig. 2). Accumulated nutrients by wastewater irrigation supported the yield of the second lettuce and produced an equivalent yield as observed in the first lettuce cropping, which received mineral NPK background fertilization. Wastewater irrigation similarly improved the biomass production of multiple crops, including maize, amaranth, roselle, and jute mallow in the tropics (Akoto-Danso et al. 2019). Akhtar et al. (2012) also reported a similar yield increase in wheat, oak, and mustard following wastewater irrigation in the temperate ecological zones.
The agronomic superiority of filtered wastewater, causing a 10% increase in crop yield compared to untreated wastewater, might have resulted from the potential release of water-soluble compounds such as K from the ash components of the biochar filter medium. In a related study, Liu et al. (2021) detected plant growth-promoting acids, such as 3-methylsalicylic acid in biochar extract, which further stimulated plant development and yield in a greenhouse experiment. Additionally, the possible removal of biosynthetic inhibitors during the filtration process, like the reduction of Fe concentration from 0.21 mg L−1 to 0.10 mg L−1 in TWW, could also explain the marginal yield improvement, as chlorophyll concentration (Table S6) was high in TWW irrigated crop similar to the study of (Gassama et al. 2015; Hajihashemi et al. 2020).
Several studies have reported yield improvement of lettuce in different soils amended with biochar (Carter et al. 2013; Steiner et al. 2018; Trupiano et al. 2017), similar to the 8% and 19% significant yield in both lettuce cycles respectively (Fig. 3). Such findings are generally explained by the improvement of primary soil fertility indicators, including SOC, pH, CEC, and K of soil by biochar addition. Contrarily, both the taproot and leaf biomass of the carrot remained unchanged with biochar addition under the same treatment conditions as the lettuce. Also, in the study of Carpenter and Nair (2014), up to 20 t ha−1 biochar amendments did not increase carrot yield. Crop type is considered an essential factor affecting biochar’s performance on yield (Haefele et al. 2011; Jeffery et al. 2017). Plant nutrient requirement and differences in the nutritional physiology of lettuce and carrot could explain their varied response to biochar addition. Principally, the monocotyledonous fibrous root of lettuce grows in multiple directions and has a higher potential to take up nutrients and water than the single taproot of carrots with fewer root hairs (Marschner 2012).
Biochar, usually depending on the feedstock, contains labile organic C and intrinsic soluble nutrients mineralized by soil microorganisms, enhancing crop production, especially in nutrient-limited soils (Mukherjee and Zimmerman 2013). The significant reduction of such properties (P, K contents, and ash component) in WB and FB through the water pretreatment process might explain the slight increase in biomass production in RB amendments compared to the soil that received pretreated biochar. Werner et al. (2018) and Hale et al. (2020) observed a similar trend in crop yield, where the soil was amended with filter biochar and water-washed biochar, respectively. In a further discussion, Werner et al. (2018) and Wang et al. (2022b) highlighted the possible contamination of FB with an active pathogen and proposed post-filtration sanitary treatment like drying, co-composting, or thermal treatment of FB prior to soil amendment.
As earlier shown by Bassirirad (2000), the tissue nutrient analysis of the carrot and lettuce reflected the levels of nutrient inputs through irrigation and soil amendment application. The macronutrient (N, P, K, and Ca) concentrations of WW and TWW irrigated crops were high corresponding to the soluble nutrient added through irrigation. A similar conclusion was established by Kaetzl et al. (2020) explaining the retention of nutrients in the biochar-filtered TWW that directly impacted the tissue concentration comparable to the WW irrigated crops. The overall low heavy metal concentration in the crop tissue (Table S7) is earlier reported by Asirifi et al. (2021a). This study using the same wastewater sources reported a minimal concentration of heavy metals because the wastewater source is purely domestic without any industrial contaminants. However, various studies made notice of potential bioaccumulation in long-term irrigation (Asirifi et al. 2021a; Kaetzl et al. 2019a)
A general improvement of tissue nutrient concentrations in both carrot and lettuce was observed in biochar-amended soil, especially for K, which is significantly increased in the untreated biochar (RB) pots. The elevated SOC, pH, CEC, and direct nutrient addition by biochar might have reduced nutrient immobility in the soil, increasing mass flow for nutrient uptake. Tissues analysis of lettuce and carrot grown on biochar-amended soil by Olszyk et al. (2020) also revealed an improved macronutrient in their tissues. In a related study, Deenik et al. (2010) indicated a slight increase in crop tissue Na, Fe, and Mn concentrations induced by biochar addition to the soil. The slightly reduced tissue N, P, and Ca concentration of WB and FB compared to RB-amended soils might be due to the loss of water-soluble biochar components through the water pretreatment process. Agreeing with the study of Werner et al. (2018), the washing and filtration process caused a substantial depletion of K content in the biochar, leading to a significant reduction of K in both crops (Figs. 5 and 6c). Lehmann et al. (2011) state that K forms a vital biochar component and directly contributes to plant nutrition. The loss of biochar-bound K through washing or during the filtration process is similar to the result of Zheng et al. (2013), who explained that up to 47% of biochar-bound K is water-soluble and is easily leached. In addition, the P and Al in the wastewater formed a crystalline AlPO4 on the surface of FB (Fig. S2) which might have reduced the impact of FB on crop nutrient uptake compared to the RB. Wang et al. (2022a) observed a similar result in a wastewater treatment and disinfection study using activated biochar.
Average concentration of nitrogen (A), phosphorus (B), potassium (C), and calcium (D) in first lettuce (lower stack) and second lettuce (upper stack) plant tissue grown on biochar amended and irrigated soil. Clean water (CW), treated wastewater (TWW) and untreated wastewater (WW) were applied as irrigation water and raw biochar (RB), filterchar (FB) and washed biochar (WB) as soil amendments. Letters indicate a significant difference of the means (MANOVA, p < 0.05) after post hoc analysis. Error bars denote the standard deviation of the mean (n = 3)
Cation exchange capacity of carrot (A) and lettuce (B) cultivated soil with biochar amendment and irrigation. Clean water (CW), treated wastewater (TWW) and untreated wastewater (WW) were applied as irrigation water and raw biochar (RB), filterchar (FB) and washed biochar (WB) as soil amendments. Ca2+, Mg2+, K+, and Na+ represent calcium, magnesium, potassium, sodium, and aluminum ions. Letters indicate a significant difference of the means (MANOVA, p < 0.05) after post hoc analysis. Error bars denote the standard deviation of the mean (n = 3)
5 Conclusion
Albeit a short irrigation term with domestic wastewater, plant-available nutrients in the wastewater agronomically contributed to a significant yield improvement of lettuce and carrot. In addition to pathogen removal, treating the wastewater with a biochar filter medium further increased its agronomic capabilities. Irrigation with biochar-filtered wastewater increased crop biomass production by up to 10%, suggesting a reduced iron (Fe) concentration and a release of labile organic compounds, for example, the ash component from the biochar to the wastewater filtrate. The biochar used for the filtration process (FB) was comparable to the water-washed biochar (WB), where both were less quality than the raw biochar (RB). Compared to the unamended control, soil amendment with untreated biochar significantly increased soil pH and biomass production of two lettuce growing cycles. The biochar’s nutrients and liming ability were reduced in the post-production treatment process and, therefore, could not increase the total biomass production of carrots. Crop irrigation with biochar-filtered wastewater on biochar-amended soil in tropical urban agroecosystems holds the potential for safe food production.
References
Adrover M, Moyà G, Vadell J (2012) Effect of treated wastewater irrigation on plant growth and biological activity in three soil types. Commun Soil Sci Plant Anal 43:1163–1180. https://doi.org/10.1080/00103624.2012.662564
Akhtar N, Arif I, Akhtar I, Nafees A (2012) Effects of city wastewater on the characteristics of wheat with varying doses of nitrogen, phosphorus, and potassium. Recent Res Sci Technol 5:18–29
Akoto-Danso EK, Manka’abusi D, Steiner C, Werner S, Häring V, Nyarko G, Marschner B, Drechsel P, Buerkert A (2019) Agronomic effects of biochar and wastewater irrigation in urban crop production of Tamale, northern Ghana. Nutr Cycl Agroecosyst 115:231–247. https://doi.org/10.1007/s10705-018-9926-6
Asirifi I, Kaetzl K, Werner S, Saba CKS, Abagale FK, Amoah P, Marschner B (2021) Pathogen and heavy metal contamination in urban agroecosystems of northern Ghana: Influence of biochar application and wastewater irrigation. J Environ Qual 50:1097–1109. https://doi.org/10.1002/jeq2.20260
Asirifi I, Werner S, Heinze S, Saba CKS, Lawson IYD, Marschner B (2021) Short-term effect of biochar on microbial biomass, respiration and enzymatic activities in wastewater irrigated soils in urban agroecosystems of the West African savannah. Agronomy 11:271. https://doi.org/10.3390/agronomy11020271
Asirifi I, Makarowsky L, Heinze S, Herre M, Werner S, Frimpong KA, Marschner B (2022) Composite of biochar and cooking ash as ameliorant for enhanced nutrient availability and microbial functions of tropical soils. Tropentag 2022 – International Research on Food Security, Natural Resource Management and Rural Development, vol 1. Cuvillier Verlag, Göttingen, p 245
Bassirirad H (2000) Kinetics of nutrient uptake by roots: responses to global change. New Phytol 147:155–169. https://doi.org/10.1046/j.1469-8137.2000.00682.x
Bhattacharya A (2018) Changing climate and resource use efficiency in plants. Academic Press, London, Waltham, MA
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Sci 59:39–46
Carpenter BH, Nair A (2014) Effect of biochar on carrot production. Iowa State University Research and Demonstration Farms Progress Reports, 2nd edn. Iowa State University Digital Press, Iowa
Carter S, Shackley S, Sohi S, Suy T, Haefele S (2013) The impact of biochar application on soil properties and plant growth of pot grown lettuce (Lactuca sativa) and cabbage (Brassica chinensis). Agronomy 3:404–418. https://doi.org/10.3390/agronomy3020404
Cataldo DA, Maroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6:71–80. https://doi.org/10.1080/00103627509366547
Chaudhary S, Vaish B, Singh RP, Prasad V (2020) Bioactive compost: an approach for managing plant growth in environmentally stressed soils. In: Rakshit A, Singh HB, Singh AK, Singh US, Fraceto L (eds) New Frontiers in Stress Management for Durable Agriculture. Springer Singapore, Singapore, pp 257–267
Deenik JL, McClellan T, Uehara G, Antal MJ, Campbell S (2010) Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci Soc Am J 74:1259–1270. https://doi.org/10.2136/sssaj2009.0115
Dutta S, Gupta B, Srivastava SK, Gupta AK (2021) Recent advances on the removal of dyes from wastewater using various adsorbents: a critical review. Mater Adv 2:4497–4531. https://doi.org/10.1039/D1MA00354B
El-Tohamy WA, El-Abagy H, Badr M, Abou-Hussein SD, Helmy YI (2011) The influence of foliar application of potassium on yield and quality of carrot (Daucus carota L.) plants grown under sandy soil conditions. Aust J Basic Appl Sci 5:171–1741
Enaime G, Baçaoui A, Yaacoubi A, Lübken M (2020) Biochar for wastewater treatment—conversion technologies and applications. Appl Sci 10:3492. https://doi.org/10.3390/app10103492
Fan R, Chen C-L, Lin J-Y, Tzeng J-H, Huang C-P, Dong C, Huang CP (2019) Adsorption characteristics of ammonium ion onto hydrous biochars in dilute aqueous solutions. Bioresour Technol 272:465–472. https://doi.org/10.1016/j.biortech.2018.10.064
Foereid B (2015) Biochar in nutrient recycling—the effect and its use in wastewater treatment. Open J. Soil Sci. 05:39–44. https://doi.org/10.4236/ojss.2015.52004
Föhse D, Claassen N, Jungk A (1991) Phosphorus efficiency of plants. Plant Soil 132:261–272. https://doi.org/10.1007/BF00010407
Frimpong KA, Abban-Baidoo E, Marschner B (2021) Can combined compost and biochar application improve the quality of a highly weathered coastal savanna soil? Heliyon 7. https://doi.org/10.1016/j.heliyon.2021.e07089
Gallego-Schmid A, Tarpani RRZ (2019) Life cycle assessment of wastewater treatment in developing countries: a review. Water Res 153:63–79. https://doi.org/10.1016/j.watres.2019.01.010
Gallego-Valero L, Moral-Parajes E, Román-Sánchez IM (2021) Wastewater treatment costs: a research overview through bibliometric analysis. Sustainability 13:5066. https://doi.org/10.3390/su13095066
Gassama UM, Puteh AB, Abd-Halim MR, Kargbo B (2015) Influence of municipal wastewater on rice seed germination, seedling performance, nutrient uptake, and chlorophyll content. J Crop Sci Biotechnol 18:9–19. https://doi.org/10.1007/s12892-014-0091-4
Germer, J.; Sauerborn, J. (2008) Ecological zones of Ghana; University of Hohenheim: Stuttgart, Germany. Available via DIALOG https://www.uni-hohenheim.de/respta/climate.php Accessed on 20 Oct 2015
Hadi MR, Karimi N (2012) The role of calcium in plants’ salt tolerance. J Plant Nutr 35:2037–2054. https://doi.org/10.1080/01904167.2012.717158
Haefele SM, Konboon Y, Wongboon W, Amarante S, Maarifat AA, Pfeiffer EM, Knoblauch C (2011) Effects and fate of biochar from rice residues in rice-based systems. Field Crops Res 121:430–440. https://doi.org/10.1016/j.fcr.2011.01.014
Hajihashemi S, Mbarki S, Skalicky M, Noedoost F, Raeisi M, Brestic M (2020) Effect of wastewater irrigation on photosynthesis, growth, and anatomical features of two wheat cultivars (Triticum aestivum L.). Water 12:607. https://doi.org/10.3390/w12020607
Hale SE, Nurida NL, Jubaedah, Mulder J, Sørmo E, Silvani L, Abiven S, Joseph S, Taherymoosavi S, Cornelissen G (2020) The effect of biochar, lime and ash on maize yield in a long-term field trial in a Ultisol in the humid tropics. Sci. Total Environ 719:137455. https://doi.org/10.1016/j.scitotenv.2020.137455
Helmecke M, Fries E, Schulte C (2020) Regulating water reuse for agricultural irrigation: risks related to organic micro-contaminants. Environ Sci Eur 32. https://doi.org/10.1186/s12302-019-0283-0
Häring V, Manka’abusi D, Akoto-Danso EK, Werner S, Atiah K, Steiner C, Lompo DJP, Adiku S, Buerkert A, Marschner B (2017) Effects of biochar, waste water irrigation and fertilization on soil properties in West African urban agriculture. Sci Rep 7:10738. https://doi.org/10.1038/s41598-017-10718-y
Jeffery S, Abalos D, Prodana M, Bastos AC, van Groenigen JW, Hungate BA, Verheijen F (2017) Biochar boosts tropical but not temperate crop yields. Environ Res Lett 12:53001. https://doi.org/10.1088/1748-9326/aa67bd
Kaetzl K, Lübken M, Gehring T, Wichern M (2018) Efficient low-cost anaerobic treatment of wastewater using biochar and woodchip filters. Water 10:818. https://doi.org/10.3390/w10070818
Kaetzl K, Lübken M, Uzun G, Gehring T, Nettmann E, Stenchly K, Wichern M (2019) On-farm wastewater treatment using biochar from local agroresidues reduces pathogens from irrigation water for safer food production in developing countries. Sci Total Environ 682:601–610. https://doi.org/10.1016/j.scitotenv.2019.05.142
Kaetzl K, Lübken M, Nettmann E, Krimmler S, Wichern M (2020) Slow sand filtration of raw wastewater using biochar as an alternative filtration media. Sci Rep 10:1229. https://doi.org/10.1038/s41598-020-57981-0
Kaetzl K, Lübken M, Uzun G, Gehring T, Nettmann E, Stenchly K, Wichern M (2019b) Improving water quality and pathogen removal using a low-cost anaerobic wastewater filtration - applicable for small scale agricultural production in developing countries. In: 12th IWA, Berlin, Germany
Koroleff F (1983) Determination of ammonia. In: Grasshoff K, Ehrhardt M, Kremling K (eds) Methods of seawater analysis, 2nd edn. Verlag Chemie, Weinheim, pp 125–139
Lehmann J, Joseph S (2015) Biochar for environmental management, 2nd edn. Routledge, London
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota – a review. Soil Biol Biochem 43:1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
Liu C, Sun B, Zhang X, Liu X, Drosos M, Li L, Pan G (2021) The water-soluble pool in biochar dominates maize plant growth promotion under biochar amendment. J Plant Growth Regul 40:1466–1476. https://doi.org/10.1007/s00344-020-10203-3
Manka’abusi D, Steiner C, Akoto-Danso EK, DJP L, Haering V, Werner S, Marschner B, Buerkert A (2019) Biochar application and wastewater irrigation in urban vegetable production of Ouagadougou, Burkina Faso. Nutr Cycling Agroecosyst 115:263–279. https://doi.org/10.1007/s10705-019-09969-0
Marschner H (ed) (2012) Marschner's mineral nutrition of higher plants, 3rd edn. Academic Press, London, Waltham, MA
Mitchell PJ, Simpson AJ, Soong R, Simpson MJ (2015) Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biol Biochem 81:244–254. https://doi.org/10.1016/j.soilbio.2014.11.017
Mizuta K, Matsumoto T, Hatate Y, Nishihara K, Nakanishi T (2004) Removal of nitrate-nitrogen from drinking water using bamboo powder charcoal. Bioresour Technol 95:255–257. https://doi.org/10.1016/j.biortech.2004.02.015
Mukherjee A, Zimmerman AR (2013) Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 193–194:122–130. https://doi.org/10.1016/j.geoderma.2012.10.002
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Ohno T, Zibilske LM (1991) Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci Soc Am J 55:892–895. https://doi.org/10.2136/sssaj1991.03615995005500030046x
Olszyk D, Shiroyama T, Novak J, Cantrell K, Sigua G, Watts D, Johnson MG (2020) Biochar affects growth and shoot nitrogen in four crops for two soils. Agrosyst Geosci Environ. https://doi.org/10.1002/agg2.20067
Pathy A, Ray J, Paramasivan B (2021) Challenges and opportunities of nutrient recovery from human urine using biochar for fertilizer applications. J Clean Prod 304. https://doi.org/10.1016/j.jclepro.2021.127019
R Core Team (2017) R: a language and environment for statistical computing. https://www.R-project.org/. Accessed 30 December 2021
Ramos FT, Dores EFC, Weber OLDS, Beber DC, Campelo JH, Maia JCS (2018) Soil organic matter doubles the cation exchange capacity of tropical soil under no-till farming in Brazil. J Sci Food Agric 98:3595–3602. https://doi.org/10.1002/jsfa.8881
Shikha FS, Sultana N, Rahman MA, Bhuiya SH, Rahman J, Akter N (2016) Effect of potassium fertilization on the growth, yield and root quality of carrot. Int J Appl Res 3:171–174
Smebye A, Alling V, Vogt RD, Gadmar TC, Mulder J, Cornelissen G, Hale SE (2016) Biochar amendment to soil changes dissolved organic matter content and composition. Chemosphere 142:100–105. https://doi.org/10.1016/j.chemosphere.2015.04.087
Steiner C, Bellwood-Howard I, Häring V, Tonkudor K, Addai F, Atiah K, Abubakari AH, Kranjac-Berisavljevic G, Marschner B, Buerkert A (2018) Participatory trials of on-farm biochar production and use in Tamale, Ghana. Agron Sustain Dev 38:12. https://doi.org/10.1007/s13593-017-0486-y
Trupiano D, Cocozza C, Baronti S, Amendola C, Vaccari FP, Lustrato G, Di Lonardo S, Fantasma F, Tognetti R, Scippa GS (2017) The effects of biochar and its combination with compost on lettuce (Lactuca sativa L.) growth, soil properties, and soil microbial activity and abundance. Int J Agron 2017:1–12. https://doi.org/10.1155/2017/3158207
Wang T, Stadler FJ, Husein DZ, Zhang D, Cai J, Wang Y, Li M, Qiang Y, Zheng J (2022) Comparative study of enhanced adsorption-photodegradation activity using activated biochar composited with Ag3PO4 or Ag6Si2O7 in wastewater treatment and disinfection: effects and mechanisms. Coll Surf A Physicochem Eng Asp 655. https://doi.org/10.1016/j.colsurfa.2022.130235
Wang T, Zheng J, Cai J, Liu Q, Zhang X (2022) Visible-light-driven photocatalytic degradation of dye and antibiotics by activated biochar composited with K+ doped g-C3N4: effects, mechanisms, actual wastewater treatment and disinfection. Sci Total Environ 839. https://doi.org/10.1016/j.scitotenv.2022.155955
Werner S, Kätzl K, Wichern M, Buerkert A, Steiner C, Marschner B (2018) Agronomic benefits of biochar as a soil amendment after its use as waste water filtration medium. Environ Pollut 233:561–568. https://doi.org/10.1016/j.envpol.2017.10.048
Xu R, Zhao A, Yuan J, Jiang J (2012) pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J Soils Sediments 12:494–502. https://doi.org/10.1007/s11368-012-0483-3
Yan B, Hou Y (2018) Effect of soil magnesium on plants: a review. Earth Environ Sci 170:22168. https://doi.org/10.1088/1755-1315/170/2/022168
Yeboah E, Asamoah G, Ofori P, Amoah B, Agyeman KOA (2020) Method of biochar application affects growth, yield and nutrient uptake of cowpea. Open Agric 5:352–360. https://doi.org/10.1515/opag-2020-0040
Zheng H, Wang Z, Deng X, Zhao J, Luo Y, Novak J, Herbert S, Xing B (2013) Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour Technol 130:463–471. https://doi.org/10.1016/j.biortech.2012.12.044
Acknowledgements
The authors would like to acknowledge the infrastructural and technical support of Sabine Frölich, Katja Gonschorek, Heidrun Kerkhoff Arshadi, Noushin, and Patrick Diehl, at the physical geography and industrial chemistry laboratory of Ruhr-Universität Bochum.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work received financial support from the German Academic Exchange Services (DAAD) and the Ruhr University Bochum, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 639 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asirifi, I., Kaetzl, K., Werner, S. et al. Biochar for Wastewater Treatment and Soil Improvement in Irrigated Urban Agriculture: Single and Combined Effects on Crop Yields and Soil Fertility. J Soil Sci Plant Nutr 23, 1408–1420 (2023). https://doi.org/10.1007/s42729-023-01132-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01132-7