Abstract
The aim of this study is to evaluate the effect of resveratrol nanocapsules (RV-NCs) by quantitative insulin sensitivity check index (QUICKI) for insulin resistance diagnosis on metabolic syndrome induction in mice. Next, the applicability of these nanocapsules (NCs) was evaluated in metabolic syndrome induced in 40 mice. RV-NCs were synthesized by interfacial deposition method and was analyzed by various methods including Zetasizer, high-performance liquid chromatograph, potentiometer and scanning electron microscopy. The analysis results show a zeta potential of − 7.15, loading efficiency of 99.54 ± 1.02% and particle size of 207 nm for RV-NC production. Collected data from mice examination were analyzed by ANOVA and Tukey criteria. The validity and the reproducibility of the QUICKI index in a population of mice were determined and found that is a significant difference between high fat diet group and the control group (p < 0.05). In addition, the result obtained by the Tukey test indicated the difference in insuline and glucose levels of these group. Also, it probably is associated with the increase in the in vivo stability, which is obtained by the effective formulation of nanocapsules for resveratrol delivery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metabolic syndromes such as obesity, dyslipidemias, and hypertension are among the signs of the pathophysiology of type II diabetes (T2D) that contribute to insulin resistance (IR). Therefore, epidemiological studies, clinical investigations and clinical practice need a precise method for evaluating insulin sensitivity and following the changes after therapeutic interposition [1]. In the regard, the validation of simple methods for quantifying insulin sensitivity in hypertensive patients is the objective of many studies [2]. It has been reported that coronary stroke and artery disease cause approximately 45% and 51% of the deaths worldwide, respectively. Symptoms of insulin resistance and obesity in metabolic syndrome are developed by resveratrol as a plant polyphenol [3]. The gold standard for measuring insulin sensitivity is the euglycaemic hyperinsulinaemic (EH) clamp technique, but this method is useless for some reasons such as high-cost and time-consuming procedure and thus is only used in research [4]. It is reported that frequently sampled intravenous glucose tolerance test (FSIGT) is a better method for direct measurement of insulin sensitivity [5]. Therefore, several methods have been developed for insulin sensitivity measurement over the last 20 years [6]. Some of these methods are based on the oral glucose tolerance test. More methods are developed based on fasting glucose and insulin values [7]. A homeostatic model (HOMA) for insulin resistance assessment based on the relationship between insulin and glucose concentration in blood was first developed in 1985 by Matthews et al. [8]. Assessment of insulin sensitivity with a new quantitative index, i.e., QUICKI, was reported in 2000 [9]. QUICKI index is derived using the inverse of the summed logarithms of the fasting insulin and glucose. This index correlates well with glucose clamp studies and is useful for measuring insulin sensitivity (IS), which is the inverse of insulin resistance. QUICKI has two main advantage: (1) it can be obtained from a fasting blood sample and (2) It is the preferred method for certain types of clinical research. QUICKI index has a significantly better correlation with glucose clamp measure of insulin sensitivity compared to the correlation between SIMM and the glucose clamp [10]. There is a positive correlation between QUICKI index and serum levels of insulin [11]. In continuing our ongoing projects on capsulation of drugs and resveratrol treatment, in the present study, we performed resveratrol NCs as an efficient tool for QUICKI index regulation and studied the effect of capsulated resveratrol in mice with metabolic syndrome by fat diet [12, 13].
2 Materials and methods
Trans-resveratrol, PCL, Span 60, and Tween 80 were purchased from Sigma Aldrich chemical companies. Fat tail with high levels of saturated fat was used to obtain high-fat diet (HFD) and the low-fat diet (LFD) was obtained from Khorasan Seeding Company.
3 Nanocapsule synthesis
RV-NC was prepared by interfacial deposition method according to the reported procedure [14]. The aqueous phase was prepared by solving polysorbate 80 (0.0380 g) in 53 mL of distilled water in a 500-mL beaker. The organic phase was also prepared using RV, PCL (0.10 g), capric triglycerides (0.330 g), and sorbitanmonostearate in 27 mL acetone by vigorous stirring in a separate beaker at 40 °C. Finally, the organic phase was added dropwise to the aqueous phase. The acetone was evaporated after 10 min. Next, the suspension was concentrated in vacuum by evaporation device and then was filtered by 8-μm filter paper. Finally, RV-NPs suspension was synthesized. The sample was diluted with MilliQ water or 0.01 μm NaCl and filtered by MILLIPORE 0.45 μm before the experiment. Average diameter and polydispersity of NCs were determined by laser light scattering method and zeta potential was measured by zeta sizer (20,101 SA, made in Japan) at 25 °C. The pH of the suspension was determined at room temperature using AL-1703 and MUNCHEN immersed electrode. SEM was used to study the surface morphology of the Nano capsule. The concentration of the loaded active substance (RV) in suspension NCs was determined using a liquid chromatography (HPLC) device equipped with a UV detector, a CLC-C8 column, and water:acetonitrile as mobile phase (40:60) with the inhibition time of 3.45 min and flow rate of 1.2 mL/min. The encapsulation efficiency was determined by the difference between the total amount of substance and free substance.
4 Biological tests
Resveratrol load as an active substance was extracted from suspension formulation by combining ultrafiltration and centrifuge. The population of this study consists of 40 male mice C57BL/6 (4-week old and weighing 20–24 g). The mice were kept under a standard condition including ad libitum access to food and water in a room controlled by a temperature of 20–24 °C and on a 12 light-dark cycle for 1 week in a laboratory to simulate environmental conditions. Ethical considerations related to animal housing and tests performed on them were in accordance with the protocol approved by the Animal Ethical Committee of Zabol University of Medical Sciences. At the end of the first week, except the control group (n = 8, LFD), other groups underwent a high-calorie diet of sheep fat (n = 32, HFD) for 12 weeks. To monitor the blood parameters, the mice were blood sampled from the eye sinus after anesthesia with ether. Parameters such as weight changes, insulin levels, glucose, and lipid profiles, were measured by FG4000 digital scale with a precision of 0.01 gr, Cayman kit with the ELISA method, ARKRAY glucometer and Kate Pars Co. by enzymatic method, respectively. Systolic and diastolic blood pressure of animals were measured using a non-invasive method with a tail-cuff sphygmomanometer (URIT, Poland). At the end of the first stage, the animals entered the treatment stage by determining the QUICKI index directly related to the levels of glucose and insulin such that to ensure the development of a metabolic syndrome model in mice. After calculating the QUICKI index and ensuring the induction of metabolic syndrome in animals, the HFD group was randomly divided into 4 groups: The groups treated with resveratrol nanocapsule (RSV-NC; 5 mg/kg/day), blank nanocapsule (B-NC; 5 mg/kg/day), free resveratrol (RSV; 100 mg/kg/day), and metformin (MET; 250 mg/kg/day).
The results were reported through AVONA as mean and standard deviation of at least three separate tests (Mean ± SD), which showed a statistically significant difference between the groups (p < 0.05). The Tukey criteria were used as a suitable criterion for distinguishing between groups.
5 Result and discussion
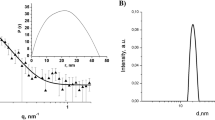
RSV-NCs and non-RSV nanocapsules (B-NC) suspensions were synthesized using biodegradable and environmentally friendly materials including poly-caprolactone (PCL) by interfacial deposition method without any additional steps. The results are presented in Table 1 and Fig. 1.
The RSV-NC and B-NC zeta potentials were measured as − 7.15 and 6.21, respectively. The negative values for the zeta potentials proved the presence of polysorbate 80 in the formulation and increased their spatial stability in water/particle interface. Suspensions of the NCs were also analyzed by DLS and the monomodal stability in the size distribution of the particles.
The polydispersity index was less than 0.3, which represents a narrow-size distribution. The SEM images taken from NCs show the spherical morphology. In addition, the average particle size analyzed by the SEM was in agreement with the data obtained from DLS. The sizes of the particles for RSV-NC and B-NC were approximately 200 nm. The formulation pH of both types of NCs was more than 4. Interestingly, the NCs were very stable such that they were stored at room temperature for 3 months without any change in the particles size and structure. The RSV content in the synthesized NCs and encapsulation efficiency was determined using the HPLC method. The encapsulation efficiency of the resveratrol in the synthesized NCs was about 99.54 ± 1.02%. This result is consistent with the latest gained reports on this formulation.
The composition of the dietary formula (HFD) and the standard dietary intake of animals (LFD) are listed in Table 2. The diet of this study contains about 30% energy from carbohydrates, about 45% from fat, and about 25% from protein, which are relatively similar to commonly used diets in different societies. The mean weight of HFD and LFD groups were compared before and after the inducing metabolic syndrome. According to the statistical results of ANOVA, there was a statistically significant difference (p value < 0.05) between the two groups. As can be seen from Fig. 2, there is a statistically significant difference between the weights of HFD (n = 32) and LFD (n = 8), probably due to the higher level of saturated fat received by the HFD group. Analyzing the obtained results by Tukey test show that HFD subgroup (RV-NC, B-NC, RV, and MET; n = 8) had higher weights, which might be because of receiving high levels of saturated fat compared to the controlled group that used a standard diet. According to the results in Fig. 2, the weight means of RV-NC, RV, and MET groups show a significant reduction during 4 weeks of treatment, of which group RV-NC showed a weight loss in a shorter time.
Since the QUICKI index is the main index of insulin resistance and is obtained directly from glucose and insulin values, these two values were compared in the study groups. The results showed a significant difference between HFD and LFD groups as shown in Fig. 3 (p < 0.05). Therefore, the highest values of glucose and insulin and the lowest QUICKI index belong to the HFD group. Based on the results of the Tukey test, glucose and insulin levels are different in five groups RV-NC, B-NC, RV, MET, and the control group. The highest levels of insulin and glucose and the lowest values of QUICKI index belong to B-NC group, which is in contrast to the controlled group. QUICKI index, insulin, and glucose values in RV-NC group were normal. Similar results were also achieved for RV and MET groups.
The results of systolic and diastolic blood pressure changes including three steps are shown in Fig. 4. By comparing the data obtained at the beginning of the study and after 4 and 12 weeks of applying the fatty diet, it can be observed that dietary fatty groups have higher systolic and diastolic blood pressure than the controlled group and the difference between HFD and LFD groups is statistically significant (p < 0.05). Tukey method was used to compare 5 subgroups of HFD group (i.e., RV-NC, B-NC, RV, and MET) after conducting the treatment phase. As can be seen, the changes in systolic and diastolic blood pressure at the end of the drug examination were significantly lower than the previous stage in RV-NC, RV, and MET groups. However, the group receiving NCs without pharmaceutical active ingredient (B-NC) had the highest systolic and diastolic blood pressure compared to other groups. The changes in systolic blood pressure are more obvious than diastolic and regulation of blood pressure by RV-NC is better than RV-NC, RV, and MET.
6 Discussion
Major risk factors for cardiovascular disease such as dyslipidemia, type II diabetes, essential hypertension, and central obesity are represented in terms of metabolic syndrome [13, 14]. Explanation of several components of the metabolic syndrome has been started about 80 years ago when Banting and Best discovered insulin [15]. Using the clamp procedure involves some limitations due to the need for establishing a steady-state equilibrium of glucose and insulin infusions. In addition, this procedure has some disadvantages such as invasiveness and the need for high costs and time. However, frequently sampled intravenous glucose tolerance test (FSIGT) is a better method for this purpose than glucose clamp and more pragmatic for direct measurement of insulin sensitivity in large studies [16, 17]. Insulin resistance is a specific characteristic of type 2 diabetes (T2D) [18, 19] and may even cause cardiovascular disease [20, 21]. Several new indexes have published in the last decades [10, 22,23,24,25,26]. HOMA index was developed in 1985 as a homeostatic model for insulin resistance assessment based on fasting glucose in blood and insulin values [26, 27]. QUICKI index was reported in 2000 as a new quantitative index for insulin sensitivity evaluation. This index compared to HOMA has a better correlation with clamp methods [10]. In line with this result, previous reports that HOMA is less reproducible than QUICKI [6, 28]. This reproducibility is due to the normalization by the logarithmic transformation of the values. It can be concluded that QUICKI may be useful in both epidemiological studies and assessment as well as insulin sensitivity follow-ups [29]. Currently, many indexes, especially the QUICKI, have has been largely validated for insulin measurement. Nevertheless, their standardization still needs to be improved [30].
The present study demonstrated that the QUICKI index correlates significantly with clamp IR before and after treatment in T2D patients. The validity and the reproducibility of the QUICKI index in a population of mice were determined and found that there is a significant difference between high fat diet (HFD) group and the control group (p < 0.05). In addition, the results obtained by the Tukey test indicated the difference in insulin and glucose levels of these groups. The highest values of QUICKI index and the lowest levels of insulin and glucose belong to the control group, in contrast to the blank nanocapsule (B-NC) group. Both the QUICKI index and the insulin and glucose levels were normal in resveratrol-nanocapsule (RV-NC) group. Polyphenolic compounds are among the most important active ingredients in edibles such as red grapes, dark chocolate, and blueberries that can be used in the treatment and regulation of insulin and blood pressure [31, 32]. Resveratrol (RSV), as a polyphenol derivative, is not commonly used by patients due to its instability and high costs [33]. Many efforts have been made to produce a new formulation to resolve these shortcomings [34, 35]. Recently, significant and promising proceedings have been taken to devise a new technology in nanostructured compounds, which have led to the development of NCs. This approach involves several advantages such as the ability to control-release system, maintaining the drug concentration in blood plasma for a long time, the possibility to develop drugs with very low doses, stability, and efficacy impressive [3]. In this study, a protected form of RSV by poly-caprolactone (PCL) coating as a biodegradable polymer was prepared.
7 Conclusion
In this study, resveratrol nanocapsules (RV-NC) are synthesized for the insulin resistance diagnosis on metabolic syndrome induction in mice. RV-NC were successfully synthesized from biodegradable materials by interfacial deposition method. The effect of RV-NC was evaluated for the first for insulin resistance (IR) diagnosis on metabolic syndrome induction time by quantitative insulin sensitivity check index (QUICKI), which is directly obtained from the calculation of the insulin and glucose content. The results proved the high efficiency and success of resveratrol nanocapsules formulation, which successfully regulated the insulin and glucose content and controlled the QUICKI index to the normal range. Our findings suggest that further studies should be conducted on the effect of RV-NC on animal and human induced type 2 diabetes, obesity, pregnancy, PCOS, premature adrenarche, hyperandrogenism, and nonalcoholic steatohepatitis models.
References
Chen H, Sullivan G, Quon MJ (2005) Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes 54(7):1914–1925
Chen H et al (2003) QUICKI is a useful index of insulin sensitivity in subjects with hypertension. Am J Physiol Endocrinol Metab 284(4):E804–E812
Shahraki A et al (2017) Resveratrol nano–capsule as an efficient tool for blood pressure regulation: a study on metabolic syndrome induced mice. Biosci Biotechnol Res Commun 10(4):623–630
Antuna-Puente B et al (2008) HOMA or QUICKI: is it useful to test the reproducibility of formulas? Diabetes Metab 34(3):294–296
Anderson RL et al (1995) Exploration of simple insulin sensitivity measures derived from frequently sampled intravenous glucose tolerance (FSIGT) tests. The insulin resistance atherosclerosis study. Am J Epidemiol 142(7):724–732
Sarafidis P et al (2007) Validity and reproducibility of HOMA–IR, 1/HOMA–IR, QUICKI and McAuley's indices in patients with hypertension and type II diabetes. J Hum Hypertens 21(9):709
Sarafidis PA, Nilsson PM (2006) The metabolic syndrome: a glance at its history. J Hypertens 24(4):621–626
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: Insulin resistance and beta–cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Hřebíček JI et al (2002) Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J Clin Endocrinol Metab 87(1):144–144
Katz A et al (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85(7):2402–2410
Kołodziejski P et al (2018) Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiol Res 67(1):45–56
Frozza RL et al (2010) Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J Biomed Nanotechnol 6(6):694–703
Organization WH (1999) Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. World health Organization, Geneva
Reilly MP, Rader DJ (2003) The metabolic syndrome: more than the sum of its parts? Circulation 108(13):1546–1551
Banting FG, Best CH (2007) The internal secretion of the pancreas. Indian J Med Res 125(3):L251
Bergman RN et al (1979) Quantitative estimation of insulin sensitivity. Am J Physiol Endocrinol Metab 236(6):E667
Bergman RN et al (1987) Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Investig 79(3):790–800
Ferrannini E (1998) Insulin resistance versus insulin deficiency in non–insulin–dependent diabetes mellitus: problems and prospects. Endocr Rev 19(4):477–490
Weyer C et al (2001) Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care 24(1):89–94
Ruige J et al (1998) Insulin and risk of cardiovascular disease: a meta–analysis. Circulation 97(10):996–1001
Haffner SM, Miettinen H (1997) Insulin resistance implications for type II diabetes mellitus and coronary heart disease. Am J Med 103(2):152–162
Raynaud E et al (1999) Revised concept for the estimation of insulin sensitivity from a single sample. Diabetes Care Alex 22:1003–1003
Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22(9):1462–1470
Belfiore F, Iannello S, Volpicelli G (1998) Insulin sensitivity indices calculated from basal and OGTT–induced insulin, glucose, and FFA levels. Mol Genet Metab 63(2):134–141
Avignon A et al (1999) Assessment of insulin sensitivity from plasma insulin and glucose in the fasting or post oral glucose-load state. Int J Obes 23(5):512
Stumvoll M et al (2001) Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 24(4):796–797
Haffner SM, Miettinen H, Stern MP (1997) The homeostasis model in the San Antonio heart study. Diabetes Care 20(7):1087–1092
Jayagopal V et al (2002) Biological variation of homeostasis model assessment-derived insulin resistance in type 2 diabetes. Diabetes Care 25(11):2022–2025
Katsuki A et al (2002) QUICKI is useful for following improvements in insulin sensitivity after therapy in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 87(6):2906–2908
Marcovina S et al (2007) Standardization of insulin immunoassays: report of the American Diabetes Association Workgroup. Clin Chem 53(4):711–716
Hennekens CH et al (1996) Lack of effect of long–term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 334(18):1145–1149
Lovejoy JC et al (2001) Relationship of dietary fat and serum cholesterol ester and phospholipid fatty acids to markers of insulin resistance in men and women with a range of glucose tolerance. Metab Clin Experim 50(1):86–92
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discovery 5(6):493
Finley JW et al (2011) Antioxidants in foods: state of the science important to the food industry. J Agric Food Chem 59(13):6837–6846
Francioso A et al (2014) Chemistry, stability and bioavailability of resveratrol. Med Chem 10(3):237–245
Acknowledgements
The findings of this study are obtained by the financial support of zabol university of medical sciences. The authors express their gratitude to Dr. Zahra Sepehri, the Deputy of Research and Technology of the university.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahraki, A., Bahadorikhalili, S., Hashemzaei, M. et al. Effects of resveratrol nanocapsules on the quantitative insulin sensitivity check index in insulin resistance: a study on metabolic syndrome induce mice. SN Appl. Sci. 2, 962 (2020). https://doi.org/10.1007/s42452-020-2759-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2759-3