Abstract
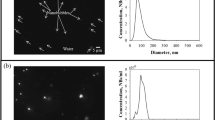
Research on bulk nanobubbles with various applications has been widely reported in the literature. However, the majority of studies are still limited to small scales with non-continuous generation processes, thus, the results may be difficult to apply on large-scale applications. In this work, a nanobubble generator was developed based on hydrodynamic cavitation using a two-chamber swirling flow nozzle that efficiently produced nanobubbles and had the potential to be applied to continuous flow systems and processes. The bulk nanobubble characteristics were evaluated according to the hydrodynamic diameter, zeta potential, and dissolved oxygen (DO) concentration based on the influence of the gas flow rate ratio (Ql/Qg), generation time, gas type, pH value, and NaCl concentration on the generated nanobubbles. The results show that oxygen and air nanobubbles smaller than 200 nm were successfully generated in pure water. The oxygen and air nanobubbles were negatively charged in pure water. The effects of pH and salt addition were similar to those during nanobubble generation using ultrasonic cavitation. In alkaline medium, nanobubbles were smaller and more stable than in acidic medium. The addition of salt to the nanobubble suspension reduced the repulsive electrostatic forces between the nanobubbles by increasing their size and decreasing their negative zeta potential. The resulting oxygen and air nanobubbles in pure water were verified to be stable for up to 10 and 5 months, respectively, without any significant changes in size or zeta potential. These results corresponded to predictions by the ionic repulsion model based on microbubble shrinkage.
Graphical abstract











Similar content being viewed by others
Abbreviations
- p B :
-
The internal pressure of the bubble, Pa.
- p rep :
-
The repulsion pressure due to hydroxyl ions adsorbed onto the bubble surface, Pa.
- p st :
-
The pressure is due to the surface tension of the liquid, Pa.
- p ∞ :
-
The pressure of the liquid or surrounding, Pa.
- ∆P :
-
Pressure difference between the internal pressure of bubble and surrounding, Pa.
- Q l :
-
Liquid flow rate, L/min.
- Q g :
-
Gas flow rate, L/min.
- Q l / Q g :
-
The flow rate ratio of the liquid relative to the gas.
- R :
-
Radius of the bubble, m.
- σ :
-
Surface tension of the liquid, N/m.
- DFM:
-
Darkfield microscopy
- DI:
-
Deionized
- DLS:
-
Dynamic light scattering
- DLVO:
-
Derjaguin–Landau–Verwey–Overbeek
- DO:
-
Dissolved oxygen, mg/L
- ELS:
-
Electrophoretic light scattering
- HDPE:
-
High-density polyethylene
- ISO:
-
International Organization for Standardization
- NTA:
-
Nanoparticle tracking analysis
- VFD:
-
Variable-frequency drive
References
Alheshibri M, Qian J, Jehannin M, Craig VSJ (2016) A history of Nanobubbles. Langmuir 32:11086–11100. https://doi.org/10.1021/acs.langmuir.6b02489
Nirmalkar N, Pacek AW, Barigou M (2018) On the existence and stability of bulk Nanobubbles. Langmuir : the ACS journal of surfaces and colloids 34:10964–10973. https://doi.org/10.1021/acs.langmuir.8b01163
Agarwal A, Ng WJ, Liu Y (2011) Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 84:1175–1180. https://doi.org/10.1016/j.chemosphere.2011.05.054
Temesgen T, Bui TT, Han M et al (2017) Micro and nanobubble technologies as a new horizon for water-treatment techniques: a review. Adv Colloid Interf Sci 246:40–51. https://doi.org/10.1016/j.cis.2017.06.011
Tanaka S, Terasaka K, Fujioka S (2020) Generation and long-term stability of ultrafine bubbles in water. Chemie Ingenieur Technik 93:1–13. https://doi.org/10.1002/cite.202000143
Gordiychuk A, Svanera M, Benini S, Poesio P (2016) Size distribution and Sauter mean diameter of micro bubbles for a Venturi type bubble generator. Exp Thermal Fluid Sci 70:51–60. https://doi.org/10.1016/j.expthermflusci.2015.08.014
Jadhav AJ, Barigou M (2020) Bulk Nanobubbles or not Nanobubbles: that is the question. Langmuir 36:1699–1708. https://doi.org/10.1021/acs.langmuir.9b03532
Rak D, Sedlák M (2020) Comment on “Bulk Nanobubbles or Not Nanobubbles: That is the Question”. Langmuir 36:15618–15621. https://doi.org/10.1021/acs.langmuir.0c01614
Hamarat S, Yeliz Y, Bozkaya ÜF (2018) Development of ultrasound-triggered and magnetic-targeted Nanobubble system for dual-drug delivery. J Pharm Sci 108:1272–1283. https://doi.org/10.1016/j.xphs.2018.10.030
Zhou T, Cai W, Yang H et al (2018) Annexin V conjugated nanobubbles: a novel ultrasound contrast agent for in vivo assessment of the apoptotic response in cancer therapy. J Control Release 276:113–124. https://doi.org/10.1016/j.jconrel.2018.03.008
Snell JR (2016) Particle formation and aggregation of a therapeutic protein in Nanobubble suspensions. J Pharm Sci 105(10):3057–3063. https://doi.org/10.1016/j.xphs.2016.06.020
Safonov VL (2013) Hydrogen nanobubbles in a water solution of dietary supplement. Colloids and Surfaces A: Physicochem Eng Aspects 436:333–336. https://doi.org/10.1016/j.colsurfa.2013.06.043
Matsuoka H, Ebina K, Tanaka H et al (2018) Administration of Oxygen Ultra-Fine Bubbles Improves Nerve Dysfunction in a rat sciatic nerve crush injury model. Int J Mol Sci 19(5):1395. https://doi.org/10.3390/ijms19051395
Lim S, Jang W, Yun S et al (2019) Amorphous germanium oxide nanobubbles for lithium-ion battery anode. Mater Res Bull 110:24–31. https://doi.org/10.1016/j.materresbull.2018.10.007
Song X (2019) Confined nanobubbles shape the surface roughness structures of thin film composite polyamide desalination membranes. J Membr Sci 582:342–349. https://doi.org/10.1016/j.memsci.2019.04.027
Azevedo A, Oliveira H, Rubio J (2019) Bulk nanobubbles in the mineral and environmental areas : updating research and applications. Adv Colloid Interf Sci 271:101992. https://doi.org/10.1016/j.cis.2019.101992
Oh SH, Han JG, Kim JM (2015) Long-term stability of hydrogen nanobubble fuel. Fuel 158:399–404. https://doi.org/10.1016/j.fuel.2015.05.072
Nakatake Y, Kisu S, Shigyo K et al (2013) Effect of nano air-bubbles mixed into gas oil on common-rail diesel engine. Energy 59:233–239. https://doi.org/10.1016/j.energy.2013.06.065
Ahmad N, Javed F, Awan JA et al (2019) Biodiesel production intensification through microbubble mediated esterification. Fuel 253:25–31. https://doi.org/10.1016/j.fuel.2019.04.173
Ebina K, Shi K, Hirao M et al (2013) Oxygen and air Nanobubble water solution promote the growth of plants, fishes, and mice. PLoS One 8:2–8. https://doi.org/10.1371/journal.pone.0065339
Reichmann F, Herath J, Mensing L, Kockmann N (2021) Gas-liquid mass transfer intensification for bubble generation and breakup in micronozzles. J Flow Chem. https://doi.org/10.1007/s41981-021-00180-3
Michailidi ED, Bomis G, Varoutoglou A et al (2020) Bulk nanobubbles: production and investigation of their formation/stability mechanism. J Colloid Interface Sci 564:371–380. https://doi.org/10.1016/j.jcis.2019.12.093
Oh SH, Kim J (2017) Generation and stability of bulk Nanobubbles. Langmuir 33(15):3818–3823. https://doi.org/10.1021/acs.langmuir.7b00510
Wang Q, Zhao H, Qi N et al (2019) Generation and stability of size-adjustable bulk Nanobubbles based on periodic pressure change. Sci Rep 9:1118. https://doi.org/10.1038/s41598-018-38066-5
Khaled A, Ahmed A, Sun C et al (2018) Generation of nanobubbles by ceramic membrane filters: the dependence of bubble size and zeta potential on surface coating, pore size and injected gas pressure. Chemosphere. https://doi.org/10.1016/j.chemosphere.2018.03.157
Ulatowski K, Sobieszuk P, Mróz A, Ciach T (2019) Stability of nanobubbles generated in water using porous membrane system. Chemical Engineering & Processing: Process Intensification 136:62–71. https://doi.org/10.1016/j.cep.2018.12.010
Yasuda K, Matsushima H, Asakura Y (2019) Generation and reduction of bulk nanobubbles by ultrasonic irradiation. Chem Eng Sci 195:455–461. https://doi.org/10.1016/j.ces.2018.09.044
Zhu J, An H, Alheshibri M et al (2016) Cleaning with bulk Nanobubbles. Langmuir 32(43):11203–11211. https://doi.org/10.1021/acs.langmuir.6b01004
Li H, Hu L, Song D, Al-Tabbaa A (2013) Subsurface transport behavior of Micro-Nano bubbles and potential applications for groundwater remediation. Int J Environ Res Public Health 11:473–486. https://doi.org/10.3390/ijerph110100473
Terasaka K, Hirabayashi A, Nishino T et al (2011) Development of microbubble aerator for waste water treatment using aerobic activated sludge. Chem Eng Sci 66:3172–3179. https://doi.org/10.1016/j.ces.2011.02.043
Hato Y (2012) Micro-bubble generator and Micro-bubble generation device. US Patent 2012/0126436 A1, May 24, 2012
Kim SB, Choi YC (2019) Nano-bubble generating apparatus. Korean Patent KR101494080 B1, Jan 31, 2019
Yasui K, Tuziuti T, Kanematsu W (2018) Mysteries of bulk nanobubbles (ultrafine bubbles); stability and radical formation. Ultrason Sonochem 48:259–266. https://doi.org/10.1016/j.ultsonch.2018.05.038
Tsuge H (2015) Micro- and Nanobubbles. CRC Press Taylor & Francis Group, Boca Raton
Jin J, Wang R, Tang J et al (2020) Dynamic tracking of bulk nanobubbles from microbubbles shrinkage to collapse. Colloids Surf A Physicochem Eng Asp 589:124430. https://doi.org/10.1016/j.colsurfa.2020.124430
Zhang M, Seddon JRT, Lemay SG (2019) Journal of colloid and Interface science nanoparticle – nanobubble interactions : charge inversion and re-entrant condensation of amidine latex nanoparticles driven by bulk nanobubbles. J Colloid Interface Sci 538:605–610. https://doi.org/10.1016/j.jcis.2018.11.110
Uchida T, Liu S, Enari M et al (2016) Effect of NaCl on the lifetime of Micro- and Nanobubbles. Nanomaterials 6:31. https://doi.org/10.3390/nano6020031
Dressaire E, Bee R, Bell DC et al (2008) Interfacial polygonal Nanopatterning of stable microbubbles. Science 320:1198–1201. https://doi.org/10.1126/science.1154601
Malvern (2015) Zeta potential - An introduction in 30 minutes. https://www.malvernpanalytical.com. Accessed 8 Feb 2021
Alam HS, Sutikno P, Soelaiman TAF, Sugiarto AT (2020) Population Balance and Computational Fluid Dynamics Modeling of Swirl Flow Microbubble Generator. 2020 International conference on sustainable energy engineering and application (ICSEEA). IEEE, Tangerang, Indonesia, pp. 1–7. https://doi.org/10.1109/ICSEEA50711.2020.9306166
Alam HS, Redhyka GG, Sugiarto AT, et al (2018) Design and performance of swirl flow microbubble generator. International journal of Engineering & Technology 7:66–69. https://doi.org/10.14419/ijet.v7i4.40.24077
Takase I, Tsutsumi H (2015) Microbubble-generating apparatus. US Patent 8,939,436 B2, Jan 27, 2015
Levitsky I, Tavor D, Gitis V (2016) Generation of two-phase air-water flow with fine microbubbles. Chemical Engineering & Technology 39:1537–1544. https://doi.org/10.1002/ceat.201500492
Alam HS, Sugiarto AT, Bahrudin, et al (2017) Micro-/Nanobubble Generator. Indonesian Patent P00201706248, Sep 18, 2017
Fu T, Ma Y, Funfschilling D, Li HZ (2009) Bubble formation and breakup mechanism in a microfluidic flow-focusing device. Chem Eng Sci 64:2392–2400. https://doi.org/10.1016/j.ces.2009.02.022
Takahashi M (2005) Potential of microbubbles in aqueous solutions: electrical properties of the gas - water Interface. J Phys Chem B 109(46):21858–21864. https://doi.org/10.1021/jp0445270
Malvern (2021) Dynamic Light Scattering: An Introduction in 30 Minutes. https://www.malvernpanalytical.com. Accessed 1 Feb 2021
Etchepare R, Oliveira H, Nicknig M et al (2017) Nanobubbles: generation using a multiphase pump, properties and features in flotation. Miner Eng 112:19–26. https://doi.org/10.1016/j.mineng.2017.06.020
Takahashi T, Miyahara T (1979) Fundamental study of bubble formation in dissolved air pressure flotation. J Chem Eng Japan 12(4):275–280. https://doi.org/10.1252/jcej.12.275
Kelsall GH, Tang S, Yurdakul S, Smith AL (1996) Electrophoretic behaviour of bubbles in aqueous electrolytes. Faraday Trans 92:3887. https://doi.org/10.1039/ft9969203887
Cho S, Kim J, Chun J, Kim J (2005) Ultrasonic formation of nanobubbles and their zeta-potentials in aqueous electrolyte and surfactant solutions. Colloids Surf A Physicochem Eng Asp 269:28–34. https://doi.org/10.1016/j.colsurfa.2005.06.063
Malvern Zetasizer ZS User Manual (English). https://www.malvernpanalytical.com. Accessed 8 Feb 2021
Ushikubo FY, Furukawa T, Nakagawa R et al (2010) Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf A Physicochem Eng Asp 361:31–37. https://doi.org/10.1016/j.colsurfa.2010.03.005
Meegoda JN, Hewage SA, Batagoda JH (2019) Application of the diffused double layer theory to Nanobubbles. Langmuir 35:12100–12112. https://doi.org/10.1021/acs.langmuir.9b01443
Yasui K (2016) Mechanism for stability of ultrafine bubbles. Japanese J Multiphase Flow 30(1):19–26. https://doi.org/10.3811/jjmf.30.19
Brennen CE (2013) Cavitation and bubble dynamics. Oxford University Press, Inc., New York
Bunkin NF, Bunkin FV (1992) Bubbstons: stable microscopic gas bubbles in very dilute electrolytic solutions. Sov Phys - JETP 24(5):271–278
Yasui K (2018) Acoustic cavitation and bubble dynamics. Springer International Publishing, Switzerland
Satpute PA, Earthman JC (2021) Hydroxyl ion stabilization of bulk nanobubbles resulting from microbubble shrinkage. J Colloid Interface Sci 584:449–455. https://doi.org/10.1016/j.jcis.2020.09.100
Acknowledgments
The author would like to thank the PhD by Research program provided by a decree from the Head of the Indonesian Institute of Sciences (Number: 149/H/2019). The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conceptualization: [Hilman Syaeful Alam]; Methodology: [Hilman Syaeful Alam]; Formal analysis and investigation: [Hilman Syaeful Alam], [Priyono Sutikno], [Tubagus Ahmad Fauzi Soelaiman], [Anto Tri Sugiarto]; Writing - original draft preparation: [Hilman Syaeful Alam]; Writing - review and editing: [Hilman Syaeful Alam], [Priyono Sutikno], [Tubagus Ahmad Fauzi Soelaiman], [Anto Tri Sugiarto]; Supervision: [Priyono Sutikno], [Tubagus Ahmad Fauzi Soelaiman], [Anto Tri Sugiarto].
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The study proposed an innovative swirling flow nozzle to generate bulk nanobubbles at a high and continuous flow rate
• The oxygen and air nanobubbles smaller than 200 nm were successfully generated and negatively charged in pure water
• The effects of pH and salt content on the nanobubble properties were similar to those reported in ultrasonic cavitation
• Bulk nanobubbles were stable for several months based on the most comprehensive data on bubble size and zeta potential
Rights and permissions
About this article
Cite this article
Alam, H.S., Sutikno, P., Soelaiman, T.A.F. et al. Bulk Nanobubbles: generation using a two-chamber swirling flow nozzle and long-term stability in water. J Flow Chem 12, 161–173 (2022). https://doi.org/10.1007/s41981-021-00208-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-021-00208-8