Abstract
Because of their potential applications, coordination polymers (CPs) are at an exalted position in the field of chemical and material science. Porous coordination polymers, popularly known as metal–organic frameworks (MOFs), have large surface area with functional pore environment, permanent porosity, tailorability in pore size, dimension and volume, which make them promising for interesting functionalities. In this review, we show how the mixed-ligand CPs/MOFs are very important in tuning the functionality of such systems and how the X-ray structure illuminates their functionalities. Here, we discuss the application of mixed-ligand functional MOFs for CO2 storage and separation by fine-tuning their pore size and dimension along with their polar pore surfaces using different functional dicarboxylates and N,N′-donor ligands. We also discuss the nature of conductivity and fabrication of Schottky barrier diode for CPs, where free organic ligands are in their pores. In addition, we also present the variation of their interesting chemical reactivity, e.g. framework-assisted in situ redox transformation.


Reproduced with permission from Ref.22 Copyright© 2011, Royal Society of Chemistry.

Reproduced with permission from Ref.23 Copyright© 2014, Royal Society of Chemistry.

Reproduced with permission from Ref.24 Copyright© 2015, Royal Society of Chemistry.

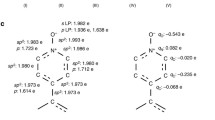
Reproduced with permission from Ref.26 Copyright© 2016, American Chemical Society.

Reproduced with permission from Ref.27 Copyright© 2016, American Chemical Society.

Reproduced with permission from Ref.30 Copyright© 2012, American Chemical Society.

Reproduced with permission from Ref.31 Copyright© 2013, American Chemical Society.

Reproduced with permission from Ref.32 Copyright© 2016, American Chemical Society.

Reproduced with permission from Ref.42 Copyright© 2014, Royal Society of Chemistry.

Reproduced with permission from Ref.43 Copyright© 2016, Royal Society of Chemistry.

Reproduced with permission from Ref.44 Copyright© 2013, Royal Society of Chemistry.

Reproduced with permission from Ref.45 Copyright© 2015, Royal Society of Chemistry.
Similar content being viewed by others
References
Desiraju GR (2014) Some themes in chemical crystallography pertinent to the Indian contribution. J Indian Inst Sci 94:1–8
Desiraju GR (1989) Crystal engineering: the design of organic solids. Elsevier, Amsterdam
Moulton B, Zaworotko MJ (2001) From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids. Chem Rev 101:1629–1658
He Y, Li B, O’Keeffec M, Chen B (2014) Multifunctional metal–organic frameworks constructed from meta-benzenedicarboxylate units. Chem Soc Rev 43:5618–5656
Zhang Z, Zhao Y, Gong Q, Lib Z, Li J (2013) MOFs for CO2 capture and separation from flue gas mixtures: the effect of multifunctional sites on their adsorption capacity and selectivity. Chem Commun 49:653–661
Zhao D, Timmons DJ, Yuan D, Zhou H-C (2011) Tuning the topology and functionality of metal–organic frameworks by ligand design. Acc Chem Res 44:123–133
Chakraborty A, Maji TK (2014) Structural diversities in metal-organic coordination polymers based on flexibility in organic spacer. J Indian Inst Sci 94:69–78
Li J-R, Sculley J, Zhou H-C (2012) Metal-organic frameworks for separations. Chem Rev 112:869–932
Bhattacharya B, Ghoshal D (2015) Selective carbon dioxide adsorption by mixed ligand porous coordination polymers. CrystEngComm 17:8388–8413
Nijem N, Wu H, Canepa P, Marti A, Balkus KJ Jr, Thonhauser T, Li J, Chabal YJ (2012) Tuning the gate opening pressure of metal–organic frameworks (MOFs) for the selective Separation of hydrocarbons. J Am Chem Soc 134:15201–15204
Dietzel PDC, Besikiotis V, Blom R (2009) Application of metal–organic frameworks with coordinatively unsaturated metal sites in storage and separation of methane and carbon dioxide. J Mater Chem 19:7362–7370
Lee JY, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT (2009) Metal–organic framework materials as catalysts. Chem Soc Rev 38:1450–1459
Givaja G, Amo-Ochoa P (2012) C.J. Go´mez-Garcı´a and F. Zamora, Electrical conductive coordination polymers. Chem Soc Rev 41:115–147
Kreno LE, Leong K, Farha OK, Allendorf M, Duyne RPV, Hupp JT (2012) Metal-organic framework materials as chemical sensors. Chem Rev 112:1105–1125
Bhattacharya B, Halder A, Paul L, Chakrabarti S, Ghoshal D (2016) Eye-catching dual-fluorescent dynamic metal–organic framework senses traces of water: experimental findings and theoretical correlation. Chem Eur J 22:14998–15005
Banerjee R, Phan A, Wang B, Knobler C, Furukawa H, O’Keeffe M, Yaghi OM (2008) High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 319:939–943
Kevitiyagala N (2009) Carbon sequestration. Science 325:1644–1645
Yeh JT, Resnik KP, Rygle K, Pennline HW (2005) Semi-batch absorption and regeneration studies for CO2 capture by aqueous ammonia. Fuel Process Technol 86:1533–1546
Horike S, Shimomura S, Kitagawa S (2009) Soft porous crystals. Nat Chem 1:695–704
Luo F, Wang M-S, Luo M-B, Sun G-M, Song Y-M, Lia P-X, Guo G-C (2012) Functionalizing the pore wall of chiral porous metal–organic frameworks by distinct –H, –OH, –NH2, –NO2, –COOH shutters showing selective adsorption of CO2, tunable photoluminescence, and direct white-light emission. Chem Commun 48:5989–5991
Wang Z, Zheng B, Liu H, Lin X, Yu X, Yi P, Yun R (2013) High-capacity gas storage by a microporous oxalamide-functionalized NbO-type metal–organic framework. Cryst Growth Des 13:5001–5006
McDonald TM, D’Alessandro DM, Krishna R, Long JR (2011) Enhanced carbon dioxide capture upon incorporation of N,N′-dimethylethylenediamine in the metal–organic framework CuBTTri. Chem Sci 2:2022–2028
Haldar R, Bonakala S, Kanoo P, Balasubramanian S, Maji TK (2014) Two 3D metal–organic frameworks of Cd(II): modulation of structures and porous properties based on linker functionalities. CrystEngComm 16:4877–4885
Bhattacharya B, Haldar R, Maity DK, Maji TK, Ghoshal D (2015) Pillared-bilayer porous coordination polymers of Zn(II): enhanced hydrophobicity of pore surface by changing the pillar functionality. CrystEngComm 17:3478–3486
Mowat JPS, Miller SR, Griffin JM, Seymour VR, Ashbrook SE, Thompson SP, Fairen-Jimenez D, Banu A-M, Duren T, Wright PA (2011) Structural chemistry, monoclinic-to-orthorhombic phase transition, and CO2 adsorption behavior of the small pore scandium terephthalate, Sc2(O2CC6H4CO2)3, and its nitro- and amino-functionalized derivatives. Inorg Chem 50:10844–10858
Maity DK, Halder A, Bhattacharya B, Das A, Ghoshal D (2016) Selective CO2 adsorption by nitro functionalized metal organic frameworks. Cryst Growth Des 16:1162–1167
Maity DK, Halder A, Ghosh S, Ghoshal D (2016) Azo functionalized 5-Nitro-1,3-benzenedicarboxylate based coordination polymers with different dimensionality and functionality. Cryst Growth Des 16:4793–4804
Dey R, Haldar R, Maji TK, Ghoshal D (2011) Three-dimensional robust porous coordination polymer with Schiff base site on the pore wall: synthesis, single-crystal-to-single-crystal reversibility, and selective CO2 adsorption. Cryst Growth Des 11:3905–3911
Bhattacharya B, Haldar R, Dey R, Maji TK, Ghoshal D (2014) Porous coordination polymers based on functionalized Schiff base linkers: enhanced CO2 uptake by pore surface modification. Dalton Trans 43:2272–2282
Bhattacharya B, Dey R, Pachfule P, Banerjee R, Ghoshal D (2013) Four 3D Cd(II)-based metal organic hybrids with different N,N′-donor spacers: syntheses, characterizations, and selective gas adsorption properties. Cryst Growth Des 13:731–739
Hijikata Y, Horike S, Sugimoto M, Inukai M, Fukushima T, Kitagawa S (2013) Pore design of two-dimensional coordination polymers toward selective adsorption. Inorg Chem 52:3634–3642
Halder A, Bhattacharya B, Dey R, Maity DK, Ghoshal D (2016) Reversible phase transformation in three dynamic mixed-ligand metal–organic frameworks: synthesis, structure, and sorption study. Cryst Growth Des 16:4783–4792
Carlucci L, Ciani G, Proserpio DM, Mitina TG, Blatov VA (2014) Entangled two dimensional coordination networks: a general survey. Chem Rev 114:7557–7580
Maity DK, Halder A, Pahari G, Haque F, Ghoshal D (2017) Hydrogen uptake by an inclined polycatenated dynamic metal–organic framework based aterial. Inorg Chem 56:713–716
Choi H-S, Suh MP (2009) Highly selective CO2 capture in flexible 3D coordination polymer networks. Angew Chem Int Ed 48:6865–6869
Sharma MK, Senkovska I, Kaskel S, Bharadwaj PK (2011) Three-dimensional porous Cd(II) coordination polymer with large one-dimensional hexagonal channels: high pressure CH4 and H2 adsorption studies. Inorg Chem 50:539–544
Thallapally PK, Grateb JW, Motkuria RK (2012) Facile xenon capture and release at room temperature using a metal–organic framework: A comparison with activated charcoal. Chem Commun 48:347–349
Liu J, Strachan DM, Thallapally PK (2014) Enhanced noble gas adsorption in Ag@MOF-74Ni. Chem Commun 50:466–468
Fernandez CA, Liu J, Thallapally PK, Strachan DM (2012) Switching Kr/Xe selectivity with temperature in a metal–organic framework. J Am Chem Soc 134:9046–9049
Wang H, Yao K, Zhang Z, Jagiello J, Gong Q, Han Y, Li J (2014) The first example of commensurate adsorption of atomic gas in a MOF and effective separation of xenon from other noble gases. Chem Sci 5:620–624
Bardeen J (1947) Surface states and rectification at a metal semi-conductor contact. Phys Rev 71:717
Bhattacharya B, Layek A, Alam MM, Maity DK, Chakrabarti S, Ray PP, Ghoshal D (2014) Cd(II) based metal–organic framework behaving as a Schottky barrier diode. Chem Commun 50:7858–7861
Bhattacharya B, Maity DK, Layek A, Jahiruddin S, Halder A, Dey A, Ghosh S, Chowdhury C, Datta A, Ray PP, Ghoshal D (2016) Multifunctional mixed ligand metal organic frameworks: X-ray structure, adsorption, luminescence and electrical conductivity with theoretical correlation. CrystEngComm 18:5754–5763
Bhattacharya B, Dey R, Maity DK, Ghoshal D (2013) Formation of three new metal organic hybrids of Cd(II) with N,N′ donor spacer: an in situ perchlorate to chloride transformation. CrystEngComm 15:9457–9464
Maity DK, Bhattacharya B, Halder A, Ghoshal D (2015) Tuned synthesis of two coordination polymers of Cd(II) using substituted bent 3-pyridyl linker and succinate: structures and their applications in anion exchange and sorption properties. Dalton Trans 44:20999–21007
Acknowledgements
We acknowledge the financial assistance of SERB, India (Grant No. SB/S1/IC-06/2014). D.K.M. acknowledges UGC for his research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maity, D.K., Ghoshal, D. Crystallography as a Path-Finding Tool to Understand Functionality in Coordination Polymers. J Indian Inst Sci 97, 261–279 (2017). https://doi.org/10.1007/s41745-017-0033-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-017-0033-5