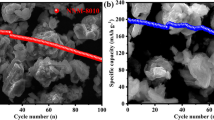
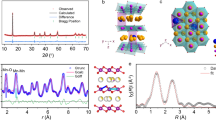
Abstract
Due to unprecedented growing demand for renewable and clean energy, as well as the shortage and uneven distribution of lithium resources, sodium-ion batteries (SIBs) are attracting increasing attention as a competitive alternative for lithium-ion batteries. Sodium ion layered oxide materials, particularly manganese-based layered oxide materials, including P2-NaxMnO2, P′2-NaxMnO2, P2-Na0.67Ni0.33Mn0.67O2, and O3-NaNi0.5Mn0.5O2, have shown high feasibility for commercialization due to their simple structures and facile synthetic methods. However, a general challenge for these materials is the poor cycling performance caused by irreversible phase transitions. Elemental doping is an effective strategy to suppress the irreversible phase transitions and improve the performance. In this paper, we review the recent progress of elemental doping in manganese-based layered oxides and explore the effects of elemental doping on crystal structure and structural evolution of manganese-based layered oxide cathode materials.

摘要
由于对可再生能源和清洁能源需求的空前增长, 以及锂资源的短缺和分布不均, 钠离子电池作为有竞争力的替代品越来越受到关注.钠离子层状氧化物材料, 特别是锰层状氧化物材料, 如P2-Na x MnO 2,P′2-NaxMnO2, P2-Na0.67Ni0.33Mn0.67O2, O3-NaNi0.5Mn0.5O2等, 具有结构简单、易于合成的优点, 因此表现出较高的商业化生产可行性. 然而,这些材料普遍面临的挑战是不可逆相变引起的不良循环性能. 元素掺杂是抑制不可逆相变, 改善材料性能的有效策略. 本文综述了锰基层状氧化物材料中元素掺杂的研究进展, 并探讨了元素掺杂对晶体结构和结构演化的影响.
Similar content being viewed by others
References
Wang L, Chen B, Ma J, et al. Reviving lithium cobalt oxide-based lithium secondary batteries-toward a higher energy density. Chem Soc Rev, 2018, 47: 6505–6602
Liu W, Oh P, Liu X, et al. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew Chem Int Ed, 2015, 54: 4440–4457
Chen M, Chou SL, Dou SX. Understanding challenges of cathode materials for sodium-ion batteries using synchrotron-based X-ray absorption spectroscopy. Batteries & Supercaps, 2019, 2: 842–851
Komaba S, Kubota K, Dahbi M, et al. Rechargeable Na-ion batteries for large format applications. In: International Renewable and Sustainable Energy Conference (IRSEC). Ouarzazate: IEEE. 2014: 651–654
Wang Y, Liu J, Lee B, et al. Ti-substituted tunnel-type Na0.44MnO2 oxide as a negative electrode for aqueous sodium-ion batteries. Nat Commun, 2015, 6: 6401
Zuo W, Yang Y. Synthesis, structure, electrochemical mechanisms, and atmospheric stability of Mn-based layered oxide cathodes for sodium ion batteries. Acc Mater Res, 2022, 3: 709–720
Ortiz-Vitoriano N, Drewett NE, Gonzalo E, et al. High performance manganese-based layered oxide cathodes: Overcoming the challenges of sodium ion batteries. Energy Environ Sci, 2017, 10: 1051–1074
Liu Y, Wang D, Li H, et al. Research progress in O3-type phase Fe/Mn/Cu-based layered cathode materials for sodium ion batteries. J Mater Chem A, 2022, 10: 3869–3888
Ren H, Zheng L, Li Y, et al. Impurity-vibrational entropy enables quasi-zero-strain layered oxide cathodes for high-voltage sodium-ion batteries. Nano Energy, 2022, 103: 107765
Cheng Z, Zhao B, Guo YJ, et al. Mitigating the large-volume phase transition of P2-type cathodes by synergetic effect of multiple ions for improved sodium-ion batteries. Adv Energy Mater, 2022, 12: 2103461
Li R, Gao J, Li J, et al. An undoped tri-phase coexistent cathode material for sodium-ion batteries. Adv Funct Mater, 2022, 32: 2205661
Zan F, Yao Y, Savilov SV, et al. Layered-tunnel structured cathode for high performance sodium-ion batteries. Funct Mater Lett, 2020, 13: 2051016
Liang X, Yu TY, Ryu HH, et al. Hierarchical O3/P2 heterostructured cathode materials for advanced sodium-ion batteries. Energy Storage Mater, 2022, 47: 515–525
Clément RJ, Billaud J, Robert Armstrong A, et al. Structurally stable Mg-doped P2-Na2/3Mn1−yMgy,O2 sodium-ion battery cathodes with high rate performance: Insights from electrochemical, NMR and diffraction studies. Energy Environ Sci, 2016, 9: 3240–3251
Xu H, Yan Q, Yao W, et al. Mainstream optimization strategies for cathode materials of sodium-ion batteries. Small Struct, 2022, 3: 2100217
Chen M, Liu Q, Wang SW, et al. High-abundance and low-cost metal-based cathode materials for sodium-ion batteries: Problems, progress, and key technologies. Adv Energy Mater, 2019, 9: 1803609
Wang C, Liu L, Zhao S, et al. Tuning local chemistry of P2 layered-oxide cathode for high energy and long cycles of sodium-ion battery. Nat Commun, 2021, 12: 2256
Zuo W, Qiu J, Liu X, et al. Highly-stable P2-Na0.67MnO2 electrode enabled by lattice tailoring and surface engineering. Energy Storage Mater, 2020, 26: 503–512
Huang Y, Zhu Y, Nie A, et al. Enabling anionic redox stability of P2-Na5/6Li1/4Mn3/4O2 by Mg substitution. Adv Mater, 2022, 34: 2105404
Yang L, Li X, Liu J, et al. Lithium-doping stabilized high-performance P2-Na0.66Li0.18Fe0.12Mn0.7O2 cathode for sodium ion batteries. J Am Chem Soc, 2019, 141: 6680–6689
Hwang JY, Myung ST, Sun YK. Quaternary transition metal oxide layered framework: O3-type Na[Ni0.32Fe0.13Co0.15Mn0.40]O2 cathode material for high-performance sodium-ion batteries J Phys Chem C, 2018, 122: 13500–13507
Rong X, Hu E, Lu Y, et al. Anionic redox reaction-induced high-capacity and low-strain cathode with suppressed phase transition Joule, 2019, 3: 612
Cao X, Li X, Qiao Y, et al. Restraining oxygen loss and suppressing structural distortion in a newly Ti-substituted layered oxide P2-Na0.66Li0.22Ti0.15Mn0.63O2. ACS Energy Lett, 2019, 4: 2409–2417
Liu X, Zuo W, Zheng B, et al. P2-Na0.67AlxMn1−xO2: Cost-effective, stable and high-rate sodium electrodes by suppressing phase transitions and enhancing sodium cation mobility. Angew Chem Int Ed, 2019, 58: 18086–18095
Dose WM, Sharma N, Pramudita JC, et al. Structure-electrochemical evolution of a Mn-rich P2 Na2/3Fe0.2Mn0.8O2 Na-ion battery cathode. Chem Mater, 2017, 29: 7416–7423
de la Llave E, Talaie E, Levi E, et al. Improving energy density and structural stability of manganese oxide cathodes for Na-ion batteries by structural lithium substitution. Chem Mater, 2016, 28: 9064–9076
Huang X, Li D, Huang H, et al. Fast and highly reversible Na+ intercalation/extraction in Zn/Mg dual-doped P2-Na0.67MnO2 cathode material for high-performance Na-ion batteries Nano Res, 2021, 14: 3531–3537
Ma P, Kang W, Wang Y, et al. Binary metal co-substituted P2-type Na0.67Mn0.7Cu0.15Ni0.15O2 microspheres as robust cathode for high-power sodium ion battery Appl Surf Sci, 2020, 529: 147105
Tie D, Gao G, Xia F, et al. Modulating the interlayer spacing and Na+/vacancy disordering of P2-Na0.67MnO2 for fast diffusion and high-rate sodium storage ACS Appl Mater Interfaces, 2019, 11: 6978–6985
Zhang X, Qiu F, Jiang K, et al. Improving the structural and cyclic stabilities of P2-type Na0.67MnO2 cathode material via Cu and Ti co-substitution for sodium ion batteries Chem Commun, 2020, 56: 6293–6296
Hwang JY, Kim J, Yu TY, et al. A new P2-type layered oxide cathode with extremely high energy density for sodium-ion batteries Adv Energy Mater, 2019, 9: 1803346
Wang K, Zhang Z, Cheng S, et al. Precipitate-stabilized surface enabling high-performance Na0.67Ni0.33−xMn0.67ZnxO2 for sodium-ion battery. eScience, 2022, 2: 529–536
Maitra U, House RA, Somerville JW, et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nat Chem, 2018, 10: 288–295
Wu Z, Ni Y, Tan S, et al. Realizing high capacity and zero strain in layered oxide cathodes via lithium dual-site substitution for sodium-ion batteries J Am Chem Soc, 2023, 145: 9596–9606
Kumakura S, Tahara Y, Kubota K, et al. Sodium and manganese stoichiometry of P2-type Na2/3MnO2 Angew Chem Int Ed, 2016, 55: 12760–12763
Ling Y, Zhou J, Guo S, et al. Copper-stabilized P′2-type layered manganese oxide cathodes for high-performance sodium-ion batteries ACS Appl Mater Interfaces, 2021, 13: 58665–58673
Chen X, Zheng S, Liu P, et al. Fluorine substitution promotes air-stability of P′2-type layered cathodes for sodium-ion batteries Small, 2023, 19: 2205789
Li S, Zhang Y, Lei K, et al. Na+/vacancy disordered manganese-based oxide cathode with ultralow strain enabled by tuning charge distribution J Mater Chem A, 2022, 10: 10391–10399
Park YJ, Choi JU, Jo JH, et al. A new strategy to build a high-performance P′2-type cathode material through titanium doping for sodium-ion batteries. Adv Funct Mater, 2019, 29: 1901912
Ren M, Zhao S, Gao S, et al. Homeostatic solid solution in layered transition-metal oxide cathodes of sodium-ion batteries. J Am Chem Soc, 2023, 145: 224–233
Billaud J, Singh G, Armstrong AR, et al. Na0.67Mn1−xMgxO2 (0 ⩽ x ⩽ 0.2): A high capacity cathode for sodium-ion batteries. Energy Environ Sci, 2014, 7: 1387–1391
Choi JU, Yoon CS, Zhang Q, et al. Understanding on the structural and electrochemical performance of orthorhombic sodium manganese oxides J Mater Chem A, 2019, 7: 202–211
Liu X, Zhong G, Xiao Z, et al. Al and Fe-containing Mn-based layered cathode with controlled vacancies for high-rate sodium ion batteries Nano Energy, 2020, 76: 104997
Zhang J, Wang W, Wang W, et al. Comprehensive review of P2-type Na2/3Ni1/3Mn2/3O2, a potential cathode for practical application of Na-ion batteries ACS Appl Mater Interfaces, 2019, 11: 22051–22066
Lu Z, Dahn JR. In situ X-ray diffraction study of P2-Na2/3[Ni1/3Mn2/3]-O2. J Electrochem Soc, 2001, 148: A1225
Wu X, Xu GL, Zhong G, et al. Insights into the effects of zinc doping on structural phase transition of P2-type sodium nickel manganese oxide cathodes for high-energy sodium ion batteries. ACS Appl Mater Interfaces, 2016, 8: 22227–22237
Feng J, Luo S, Wang J, et al. Stable electrochemical properties of magnesium-doped Co-free layered P2-type Na0.67Ni0.33Mn0.67O2 cathode material for sodium ion batteries. ACS Sustain Chem Eng, 2022, 10: 4994–5004
Yang L, Luo S, Wang Y, et al. Cu-doped layered P2-type Na0.67Ni0.33−xCuxMn0.67O2 cathode electrode material with enhanced electrochemical performance for sodium-ion batteries Chem Eng J, 2021, 404: 126578
Yang Q, Wang PF, Guo JZ, et al. Advanced P2-Na2/3Ni1/3Mn7/12Fe1/12O2 cathode material with suppressed P2–O2 phase transition toward high-performance sodium-ion battery ACS Appl Mater Interfaces, 2018, 10: 34272–34282
Pahari D, Puravankara S. On controlling the P2–O2 phase transition by optimal Ti-substitution on Ni-site in P2-type Na0.67Ni0.33Mn0.67O2 (NNMO) cathode for Na-ion batteries J Power Sources, 2020, 455: 227957
Wu X, Guo J, Wang D, et al. P2-type Na0.66Ni0.33−xZnxMn0.67O2 as new high-voltage cathode materials for sodium-ion batteries J Power Sources, 2015, 281: 18–26
Wang K, Wan H, Yan P, et al. Dopant segregation boosting high-voltage cyclability of layered cathode for sodium ion batteries Adv Mater, 2019, 31: 1904816
Huang Q, Wang M, Zhang L, et al. Shear-resistant interface of layered oxide cathodes for sodium ion batteries Energy Storage Mater, 2022, 45: 389–398
Peng B, Chen Y, Wang F, et al. Unusual site-selective doping in layered cathode strengthens electrostatic cohesion of alkali-metal layer for practicable sodium-ion full cell. Adv Mater, 2022, 34: e2103210
Lee E, Lu J, Ren Y, et al. Layered P2/O3 intergrowth cathode: Toward high power Na-ion batteries. Adv Energy Mater, 2014, 4: 1400458
Komaba S, Nakayama T, Ogata A, et al. Electrochemically reversible sodium intercalation of layered NaNi0.5Mn0.5O2 and NaCrO2. ECS Trans, 2009, 16: 43–55
Yuan DD, Wang YX, Cao YL, et al. Improved electrochemical performance of Fe-substituted NaNi0.5Mn0.5O2 cathode materials for sodium-ion batteries ACS Appl Mater Interfaces, 2015, 7: 8585–8591
Leccardi F, Nodari D, Spada D, et al. Synergistic effect of polymorphs in doped NaNi0.5Mn0.5O2 cathode material for improving electrochemical performances in Na-batteries Electrochem, 2021, 2: 335–346
Yao HR, Lv WJ, Yin YX, et al. Suppression of monoclinic phase transitions of O3-type cathodes based on electronic delocalization for Na-ion batteries. ACS Appl Mater Interfaces, 2019, 11: 22067–22073
Zheng S, Zhong G, McDonald MJ, et al. Exploring the working mechanism of Li+ in O3-type NaLi0.1Ni0.35Mn0.55O2 cathode materials for rechargeable Na-ion batteries. J Mater Chem A, 2016, 4: 9054–9062
Meng Y, An J, Chen L, et al. A NaNi0.5Mn0.5SnxO2 cathode with anti-structural deformation enhancing long lifespan and super power for a sodium ion battery Chem Commun, 2020, 56: 8079–8082
Wang PF, Yao HR, Liu XY, et al. Ti-substituted NaNi0.5Mn0.5−xTi cathodes with reversible O3–P3 phase transition for high-performance sodium-ion batteries. Adv Mater, 2017, 29: 1700210
Zhang X, Zhou YN, Yu L, et al. Suppressing multiphase transitions of an O3-NaNi0.5Mn0.5O2 cathode by iron and magnesium co-doping towards sodium-ion batteries Mater Chem Front, 2021, 5: 5344–5350
Kubota K, Fujitani N, Yoda Y, et al. Impact of Mg and Ti doping in O3 type NaNi1/2Mn1/2O2 on reversibility and phase transition during electrochemical Na intercalation J Mater Chem A, 2021, 9: 12830–12844
Yao HR, Wang PF, Gong Y, et al. Designing air-stable O3-type cathode materials by combined structure modulation for Na-ion batteries J Am Chem Soc, 2017, 139: 8440–8443
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22109091 and 91963113).
Author information
Authors and Affiliations
Contributions
Author contributions Jiang H wrote the paper; Jiang H and Qian G prepared the figures and tables; Liu WD revised the manuscript; Chen Y provided the overall concept and revised the manuscript. All authors participated in the discussion.
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Supplementary information Supporting data are available in the online version of the paper.
Haoran Jiang obtained a Bachelor’s degree from Qingdao University of Science and Technology. She is currently a Master candidate of materials science and engineering at Tianjin University. Her research direction is energy storage SIBs.
Yanan Chen is a professor at the School of Materials Science and Engineering, Tianjin University. He received his joint PhD degree from the University of Science and Technology Beijing/University of Maryland in 2017. He was an Advanced Innovative Fellow at Tsinghua University before joining Tianjin University. His research mainly focuses on nanomaterials, devices, and systems for advanced energy storage and conversion, including nanomaterials synthesis and nanomanufacturing, emerging energy storage Li-ion and beyond, catalysis, and Cryo-EM.
Rights and permissions
About this article
Cite this article
Jiang, H., Qian, G., Liu, R. et al. Effects of elemental doping on phase transitions of manganese-based layered oxides for sodium-ion batteries. Sci. China Mater. 66, 4542–4549 (2023). https://doi.org/10.1007/s40843-023-2617-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-023-2617-5