Abstract
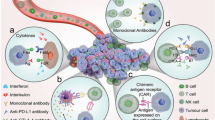
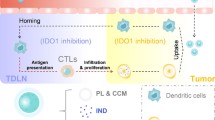
Immunotherapy has shown promising potential in cancer therapy; however, poor delivery by nanocarriers and insufficient immune response in tumors have severely impeded its clinical application. To overcome these disadvantages, a site-specific and active transcellular drug delivery system was developed herein for chemotherapy-enhanced immunotherapy. When arriving at the tumor site, the matrix metallopeptidase 2 (MMP2)-responsive shell detached from the nanosystem, releasing positively charged cores. The cationic surface of the inner cores induced adsorption-meditated transcytosis, which facilitated transendothelial transportation and transcellular drug delivery into distal tumor cells. PD-L1 antibody and chemotherapeutic drugs were loaded in the outer layer and inner cores of the nanosystem, respectively, to be precisely delivered to target sites, thereby achieving synchronized delivery and site-oriented release of different anticancer agents. PD-L1 antibody released in the tumor microenvironment effectively blocked the binding of PD-L1 to its receptors on the T cell surface. Oxaliplatin and indoximod co-delivered in the cationic cores can induce immunogenic cell death and attenuate the immunosuppressive effect throughout the tumor tissues, recruiting a large amount of T cells and further enhancing the immunotherapy. The resulting synergistic antitumor response could not only efficiently inhibit the growth of primary tumors, but also help prevent metastasis of primary tumor to distant sites. This study offers a novel nano-enabled strategy for chemo-immunotherapy in immunosuppressive tumors.

摘要
目前免疫治疗已经显示出了极大的肿瘤治疗潜力, 然而纳米载体较低的转运能力以及肿瘤免疫响应性低等问题严重阻碍了免疫治疗的临床应用. 为了解决这些问题, 本课题研制了一种具有主动穿细胞转运能力的定向纳米载体用于化疗增强的免疫治疗. 当该纳米载药系统到达肿瘤部位, 金属基质蛋白酶2响应的纳米外壳崩解, 释放出带有正电荷的纳米内核. 正电荷促使纳米内核产生吸附介导的胞吞, 进而促进纳米载药系统的跨血管内皮细胞运输和跨细胞药物递送, 并最终将药物递送到远端肿瘤细胞中. PD-L1抗体和化疗药物分别被载于纳米载体的外壳和内核中进行精准递送, 从而达到对具有不同治疗靶点的药物同步递送、定向释药的目的. 肿瘤微环境中释放的PD-L1抗体作用于T细胞表面的特异性受体, 阻断了T细胞与肿瘤细胞的结合. 正电荷纳米内核同步运载奥沙利铂和吲哚美辛进入深层肿瘤细胞, 在整个肿瘤组织中引发免疫原性死亡、逆转免疫抑制作用, 招募大量的T细胞并最终增强免疫治疗疗效. 本研究中所构建的纳米载药体系不仅能够抑制原位瘤生长, 还能够阻止肿瘤的转移. 这为化疗增强的免疫治疗提供了一种新的纳米递送方案, 提高了免疫治疗在免疫响应率低的肿瘤中的疗效.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Doroshow DB, Bhalla S, Beasley MB, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol, 2021, 18: 345–362
Wan C, Keany MP, Dong H, et al. Enhanced efficacy of simultaneous PD-1 and PD-L1 immune checkpoint blockade in high grade serous ovarian cancer. Cancer Res, 2020, 81: 158–173
Yu JX, Hodge JP, Oliva C, et al. Trends in clinical development for PD-1/PD-L1 inhibitors. Nat Rev Drug Discov, 2020, 19: 163–164
Park SE, Lee SH, Ahn JS, et al. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thoracic Oncol, 2018, 13: 106–111
Su Z, Xiao Z, Wang Y, et al. Codelivery of anti-PD-1 antibody and paclitaxel with matrix metalloproteinase and pH dual-sensitive micelles for enhanced tumor chemoimmunotherapy. Small, 2020, 16: 1906832
Hu C, He X, Chen Y, et al. Metformin mediated PD-L1 downregulation in combination with photodynamic-immunotherapy for treatment of breast cancer. Adv Funct Mater, 2021, 31: 2007149
Ferrara R, Naigeon M, Auclin E, et al. Circulating T-cell immunosenescence in patients with advanced non-small cell lung cancer treated with single-agent PD-1/PD-L1 inhibitors or platinum-based chemotherapy. Clin Cancer Res, 2021, 27: 492–503
Wang C, Shi X, Song H, et al. Polymer-lipid hybrid nanovesicle-enabled combination of immunogenic chemotherapy and RNAi-mediated PD-L1 knockdown elicits antitumor immunity against melanoma. Biomaterials, 2021, 268: 120579
Chen Y, Ma H, Wang W, et al. A size-tunable nanoplatform: Enhanced MMP2-activated chemo-photodynamic immunotherapy based on biodegradable mesoporous silica nanoparticles. Biomater Sci, 2021, 9: 917–929
Timaner M, Kotsofruk R, Raviv Z, et al. Microparticles from tumors exposed to radiation promote immune evasion in part by PD-L1. Oncogene, 2020, 39: 187–203
Zheng J, Sun J, Chen J, et al. Oxygen and oxaliplatin-loaded nanoparticles combined with photo-sonodynamic inducing enhanced immunogenic cell death in syngeneic mouse models of ovarian cancer. J Control Release, 2021, 332: 448–459
Gao J, Wang WQ, Pei Q, et al. Engineering nanomedicines through boosting immunogenic cell death for improved cancer immunotherapy. Acta Pharmacol Sin, 2020, 41: 986–994
Kesarwani P, Prabhu A, Kant S, et al. Tryptophan metabolism contributes to radiation-induced immune checkpoint reactivation in glioblastoma. Clin Cancer Res, 2018, 24: 3632–3643
Volarevic V, Zdravkovic N, Harrell CR, et al. Galectin-3 regulates indoleamine-2,3-dioxygenase-dependent cross-talk between colon-infiltrating dendritic cells and T regulatory cells and may represent a valuable biomarker for monitoring the progression of ulcerative colitis. Cells, 2019, 8: 709
Wang J, Li Z, Wang Z, et al. Nanomaterials for combinational radioimmuno oncotherapy. Adv Funct Mater, 2020, 30: 1910676
Long GV, Dummer R, Hamid O, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol, 2019, 20: 1083–1097
Breakstone R. Colon cancer and immunotherapy—Can we go beyond microsatellite instability? Transl Gastroenterol Hepatol, 2021, 6: 12
Muller AJ, Manfredi MG, Zakharia Y, et al. Inhibiting IDO pathways to treat cancer: Lessons from the ECHO-301 trial and beyond. Semin Immunopathol, 2019, 41: 41–48
Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol, 2019, 12: 92
Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med, 2019, 381: 2020–2031
Luo L, Li L, Cong C, et al. Catalase-like nanosystem for interlocking trimodal cancer therapy with hypoxia relief. Sci China Mater, 2021, 64: 1021–1034
Xue X, Li J, Fan Y, et al. Gene silencing-mediated immune checkpoint blockade for tumor therapy boosted by dendrimer-entrapped gold nanoparticles. Sci China Mater, 2021, 64: 2045–2055
Cheng G, Yin C, Tu H, et al. Controlled co-delivery of growth factors through layer-by-layer assembly of core-shell nanofibers for improving bone regeneration. ACS Nano, 2019, 13: 6372–6382
Liu P, Liu X, Cheng Y, et al. Core-shell nanosystems for self-activated drug-gene combinations against triple-negative breast cancer. ACS Appl Mater Interfaces, 2020, 12: 53654–53664
Lin F, Chen J, Lee M, et al. Multi-responsive ibuprofen-imprinted core-shell nanocarriers for specific drug recognition and controlled release. ACS Appl Nano Mater, 2020, 3: 1147–1152
Albinali KE, Zagho MM, Deng Y, et al. A perspective on magnetic core-shell carriers for responsive and targeted drug delivery systems. IJN, 2019, Volume 14: 1707–1723
Yang H, Tong Z, Sun S, et al. Enhancement of tumour penetration by nanomedicines through strategies based on transport processes and barriers. J Control Release, 2020, 328: 28–44
Zinger A, Koren L, Adir O, et al. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano, 2019, 13: 11008–11021
Yuan F, Leunig M, Huang SK, et al. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res, 1994, 54: 3352–3356
Huo D, Jiang X, Hu Y. Recent advances in nanostrategies capable of overcoming biological barriers for tumor management. Adv Mater, 2019, 32: 1904337
Zhou Q, Dong C, Fan W, et al. Tumor extravasation and infiltration as barriers of nanomedicine for high efficacy: The current status and transcytosis strategy. Biomaterials, 2020, 240: 119902
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol, 2015, 33: 941–951
Yang X, Hu C, Tong F, et al. Tumor microenvironment-responsive dual drug dimer-loaded PEGylated bilirubin nanoparticles for improved drug delivery and enhanced immune-chemotherapy of breast cancer. Adv Funct Mater, 2019, 29: 1901896
Sanmamed MF, Chen L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J, 2014, 20: 256–261
Ruan S, Xie R, Qin L, et al. Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with anti-PD-L1 antibody for improved glioma treatment. Nano Lett, 2019, 19: 8318–8332
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21874078 and 22074072), Taishan Young Scholar Program of Shandong Province (tsqn20161027), the Natural Science Foundation of Shandong Province (ZR2019BH032), the People’s Livelihood Science and Technology Project of Qingdao (166257nsh and 173378nsh), and the First-Class Discipline Project of Shandong Province.
Author information
Authors and Affiliations
Contributions
Zhang M conceived the idea of this study; Zhang M and Wang W designed the experiments; Zhang M, Wang W and Ma H performed the experiments and data analysis; Zhang M, Yu B, Cong H and Shen Y contributed to writing, drafting, and editing of this article.
Corresponding author
Additional information
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Supporting data are available in the online version of the paper.
Min Zhang received her PhD degree in 2017 from China Pharmaceutical University. In 2017, she joined Qingdao University as an associate professor. Her current research interest is the precise delivery of anticancer drugs.
Hailin Cong received his PhD degree from Peking University in 2004. After obtaining his PhD degree, he worked as a postdoctoral fellow at University of Wyoming and University of California (2004–2009). Thereafter, he joined Qingdao University as a full professor. His current interest of research focuses on functional polymer materials, bio-nanomaterials, special fibers and rubber, and plastic materials.
Rights and permissions
About this article
Cite this article
Zhang, M., Wang, W., Ma, H. et al. A site-oriented nanosystem for active transcellular chemo-immunotherapy to prevent tumor growth and metastasis. Sci. China Mater. 65, 1391–1402 (2022). https://doi.org/10.1007/s40843-021-1846-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-021-1846-8