Abstract
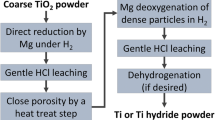
Titanium metallic powder (2.98 wt% O) of irregular and semi-spherical particles sizes ranging between 0.5 and 3.5 µm was obtained by magnesiothermic reduction of TiO2 and a leaching purification process. Magnesiothermic reduction experiments were carried out to evaluate the influence of temperature and molar ratio of Mg/TiO2. A rotary tube reactor in three different configurations was used to promote solid–liquid and solid–gas model reaction. The best configuration resulted when solid–gas model reaction was promoted. Different mixtures of acid as 6M HCl, 3M HCl, 0.55M HCl plus a mixture of 8 HCl% + 3 HNO3% were used to evaluate the purification of solid titanium metal by dissolution of Mg, MgO, Ni, Fe, magnesium titanates, and titanium oxides.











Similar content being viewed by others
References
Prasad S, Ehrensberger M, Gibson MP et al (2015) Biomaterial properties of titanium in dentistry. J Oral Biosci 57:192–199
Yang Y, Zhang C, Dai Y, Luo J (2017) Tribological properties of titanium alloys under lubrication of SEE oil and aqueous solutions. Tribol Int 109:40–47. https://doi.org/10.1016/j.triboint.2016.11.040
Hurless BE, Froes FH (2002) Lowering the cost of titanium. AMPTIAC Q. 6:3–9
Norgate TE, Wellwood G (2006) The potential applications for titanium metal powder and their life cycle impacts. JOM 58:58–63. https://doi.org/10.1007/s11837-006-0084-y
Kraft EH (2004) Summary of emerging titanium cost reduction technologies. Report by EHK Technology Vancouver, WA Study for US DofE and ORNL/Subcontract 4000023694
Barnes J, Kingsbury A, Bono E (2016) Does "low cost" titanium powder yield low cost titanium parts? In: PowderMet 2016 international conference on powder metallurgy. Boston
Faller K, Froes FH (2001) The use of titanium in family automobiles: current trends. JOM 53:27–28. https://doi.org/10.1007/s11837-001-0143-3
Ashraf Imam M, Froes FHS (2010) Low cost titanium and developing applications. JOM 62:17–20. https://doi.org/10.1007/s11837-010-0069-8
Froes FHS, Gungor MN, Ashraf Imam M (2007) Cost-affordable titanium: The component fabrication perspective. JOM 59:28–31. https://doi.org/10.1007/s11837-007-0074-8
German R (2009) Titanium powder injection moulding: a review of the current status of materials, processing, properties and applications. Powder Inject Mould Int 3:21–37. https://doi.org/10.1093/hmg/ddr404
Dutta B, Froes F (2015) The additive manufacturing (AM) of titanium alloys. In: Advanced materials research, pp 447–468
Froes FHS (2012) Titanium powder metallurgy: a review—part 1. Adv Mater Process. 170:16–22
Bolzoni L, Ruiz-Navas EM, Neubauer E, Gordo E (2012) Inductive hot-pressing of titanium and titanium alloy powders. Mater Chem Phys 131:672–679. https://doi.org/10.1016/j.matchemphys.2011.10.034
Cui C, Hu B, Zhao L, Liu S (2011) Titanium alloy production technology, market prospects and industry development. Mater Des - MATER Des 32:1684–1691. https://doi.org/10.1016/j.matdes.2010.09.011
Vlad AIO (2008) Innovative powder metallurgy process for producing low cost titanium alloy component. In: Titanium 2008, Las Vegas
Capus J (2017) Titanium powder developments for AM: a round-up. Met Powder Rep 72:384–388. https://doi.org/10.1016/j.mprp.2017.11.001
Neotiss AB (2017) Global trends in industrial market. In: Titanium Europe 20, Amsterdam
Cain KJ (2016) Industrial titanium demand forecast 2016. In: Titanium USA 2016, Boston
Gopienko VG, Neikov OD (2009) Chapter 14: production of titanium and titanium alloy powders. Handbook of non-ferrous metal powders: technologies and applications. Elsevier, Oxford, pp 314–323
Whittaker D, Froes FH (2015) 30—future prospects for titanium powder metallurgy markets BT—titanium powder metallurgy. Titanium Powder Metallurgy. Butterworth-Heinemann, Boston, pp 579–600
Suzuki RO, Ono K, Teranuma K (2003) Calciothermic reduction of titanium oxide and in-situ electrolysis in molten CaCl2. Metall Mater Trans B 34:287–295. https://doi.org/10.1007/s11663-003-0074-1
Suzuki RO, Inoue S (2003) Calciothermic reduction of titanium oxide in molten CaCl2. Metall Mater Trans B 34:277–285. https://doi.org/10.1007/s11663-003-0073-2
Fray DJ (2001) Emerging molten salt technologies for metals production. JOM 53:27–31. https://doi.org/10.1007/s11837-001-0052-5
Mohandas KS, Fray D (2004) FFC Cambridge process and removal of oxygen from metal-oxygen systems by molten salt electrolysis: an overview. Trans Indian Inst Met 57:579–592
Hogan L, McGinn E, Kendall R (2008) Research and development in titanium - implications for a titanium metal industry in Australia. ABARE, Canberra
Borys S, Anderson RP, Benish A, Jacobsen L, Ernst W, Kogut D, Lyssenko T (2005) Development status of the armstrong process for production of low cost titanium powder. In: Aeromat 2005, Orlando
Okabe TH, Oda T, Mitsuda Y (2004) Titanium powder production by preform reduction process (PRP). J Alloys Compd 364:156–163. https://doi.org/10.1016/S0925-8388(03)00610-8
Trzcinski M (2006) The race is on for commercialization of titanium powder. Light Met Age 6:30–33
Zhang Y, Fang ZZ, Xia Y et al (2017) Hydrogen assisted magnesiothermic reduction of TiO2. Chem Eng J 308:299–310. https://doi.org/10.1016/j.cej.2016.09.066
Nersisyan HH, Won HI, Won CW et al (2014) Direct magnesiothermic reduction of titanium dioxide to titanium powder through combustion synthesis. Chem Eng J 235:67–74. https://doi.org/10.1016/j.cej.2013.08.104
Kan X, Ding J, Zhu H et al (2017) Low temperature synthesis of nanoscale titanium nitride via molten-salt-mediated magnesiothermic reduction. Powder Technol 315:81–86. https://doi.org/10.1016/j.powtec.2017.03.042
Nersisyan HH, Won HI, Won CW et al (2013) Combustion synthesis of porous titanium microspheres. Mater Chem Phys 141:283–288. https://doi.org/10.1016/j.matchemphys.2013.05.012
Bolívar R, Friedrich B (2009) Synthesis of titanium via magnesiothermic reduction of TiO2 (pigment). Proc Eur Metall Conf EMC 2009:1235–1254
Oosterhof C, Reitz J, Bolivar RBF (2010) Potentiale alternativer Herstellungskonzepte für Titanmetall und Titanlegierungen. In: 44. Metallurgische Seminar des Fachausschusses für Metallurgische, pp 131–162
Acknowledgements
The research was financially supported by the DAAD-scholarship Ph.D. Program, Universidad de Pamplona-Ph.D. Program, and by IME-RWTH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Julie M. Schoenung.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bolivar, R., Friedrich, B. Magnesiothermic Reduction from Titanium Dioxide to Produce Titanium Powder. J. Sustain. Metall. 5, 219–229 (2019). https://doi.org/10.1007/s40831-019-00215-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-019-00215-z