Abstract
Since FY2008, Japanese steel manufacturers (four blast furnace steel manufacturers and one engineering company) have been promoting projects for the “Development of technologies for an environmentally harmonized steelmaking process” under COURSE50 (CO2 Ultimate Reduction in Steelmaking process by innovative technology for cool Earth 50), a national project commissioned by the New Energy and Industrial Technology Development Organization. The goal of COURSE50 is to establish technologies which contribute to a mitigation of approximately 30 % in CO2 emissions at integrated steel plants by 2050. Two kinds of technologies are being investigated: (a) Intensified hydrogen reduction of iron ore using coke oven gas to curb carbon input in blast furnaces, and (b) Sequestration of CO2 in the blast furnace gas through the chemical absorption method and physical adsorption method by the effective utilization of unused waste heat in the integrated steel plants. In the case of (a), the main challenge is the conservation of global and local heat balances in the blast furnace. In the case of (b), the main task is improving the energy consumption of chemical absorption and physical adsorption by improving the agents and materials as well as the process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As an activity of the United Nations Framework Convention on Climate Change, the twenty-first session of the Conference of the Parties (COP21) was held in December 2015. Requirements for CO2 emissions mitigation are becoming stricter every year throughout the world. According to the statistics [1] on the green house gas emissions in Japan, the steel industry accounts for 12.4 % of the total CO2 emissions in Japan. Therefore, the steel industry in Japan needs to reduce its CO2 emissions, and given this, it is necessary to reduce CO2 emissions from the overall steel industry activities in Japan.
Since the oil crisis, the Japanese steel industry has been working actively on energy saving measures for the purpose of cost reduction. It has been reported that the energy efficiency of the Japanese steel industry is currently at the world’s highest level [2]. In other words, in Japanese steel mills, there is less room for further energy savings through existing technologies, so the development of innovative steelmaking process technologies to achieve further CO2 reductions is desired.
The blast furnace-BOF method [3] is a major process in the steel industry that uses carbon derived from coal to reduce the iron ore. CO2 emissions per unit of steel are larger than the electric arc furnace (EAF) process [4] based on scrap melting. In the EAF process, since contamination occurs due to impurities, such as copper, it is difficult to ensure the quality required for high-quality steel products. Meanwhile, although direct reduction processes such as MIDREX [5] using natural gas can mitigate CO2 emissions, the production capacity of the DR process does not reach the level of blast furnaces. Considering the required productivity and time schedule until FY2030, which was determined based on the plan to reline the commercial blast furnaces through FY2050, the blast furnace-BOF process has been selected as the base process.
Therefore, Kobe Steel, Ltd., JFE Steel Corporation, Nippon Steel & Sumitomo Metal Corporation, Nippon Steel & Sumikin Engineering Co. Ltd., and Nisshin Steel Co., Ltd. have been executing an R&D program on the “Development of technologies for an environmentally harmonized steelmaking process” under COURSE50 (CO2 Ultimate Reduction in Steelmaking process by innovative technology for cool Earth 50), a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO) [6]. This project started in July 2008 as a 5-year fundamental R&D project (Step 1) and has currently evolved into a 5-year integrated R&D project (Step 2) which includes iron ore reduction by hydrogen and CO2 separation and recovery.
Table 1 shows the approach of several major CO2 mitigation methods. In view of removing carbon, three items can be listed: (1) utilization of hydrogen, (2) direct utilization of electricity, and (3) utilization of C-Neutral reducing agents. Here (2) hydrometallurgical electrolysis method is not suitable from the perspective of productivity, and (3) utilization of C-Neutral reducing agents is limited in terms of supply capacity, which is restricted by the collection and transportation of such agents. In the case of (1) utilization of hydrogen, indirect utilization of electricity (electricity applied to hydrogen production) is not feasible as the low hydrogen supply rate does not match the high production rate of the blast furnace.
Use of natural gas and by-product Coke Oven Gas (COG) has no particular fatal problems in terms of supply capacity and can be supplied at rates consistent with the high productivity of the blast furnace. After comparing the specific CO2 emissions from natural gas and COG, COG was selected as a candidate.
On the other hand, technology development for applying COG to iron ore reduction has limitations from the perspective of the endothermic reaction of the hydrogen reduction and the energy balance in integrated steel plants, and this is the reason intensified hydrogen reduction in the blast furnace was selected as an innovative technology development of steel industry, which forms the core of COURSE50.
The suppression of direct reduction by intensified indirect reduction leads to a decrease in carbon input. In COURSE50, to promote effective indirect reduction, intensified hydrogen reduction in place of CO reduction is applied within the appropriate heat balance in the blast furnace. The intensified hydrogen reduction can be attained by COG injection or the injection of reformed COG. Moreover, in addition to the decrease in carbon input by intensified hydrogen reduction, the sequestration of CO2 from the blast furnace gas is applied to decrease the output of carbon.
In the following sections, we provide an overview of the current major progress that has been achieved.
The Aim of This Project
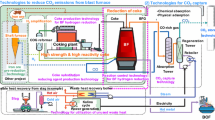
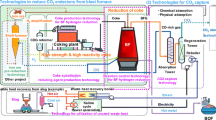
Figure 1 shows an outline of the COURSE50 project. The main research activities consist of two parts. The former is the development of technologies to mitigate CO2 emissions through intensified hydrogen reduction of iron ore in the blast furnace.
Outline of COURSE50 concept [7]
-
(1)
Reaction control technology for intensified hydrogen reduction.
-
(2)
Hydrocarbon in COG reforming technology for enhancing the use of hydrogen.
-
(3)
Coke improvement technology for low coke ratio operation in order to compensate for the strength of the charged coke in the blast furnace, whose specific consumption is reduced.
The latter is the development of CO2 sequestration technologies by the chemical absorption method and physical adsorption method to remove CO2 from the blast furnace gas using waste exhaust heat, which currently has low economic value and use at the steel plant.
The technology development goals for the mitigation of CO2 emissions using both technologies are about 30 % of CO2 emissions from an integrated steel plant.
Thus, 30 % CO2 emissions reduction will be achieved through:
-
Approx. 10 % mitigation by lowering CO2 emissions from the blast furnace. This percentage has been determined by the available COG and increase in the reaction efficiency of CO and H2 reductions.
-
Approx. 20 % mitigation through the sequestration of CO2 in BFG. This percentage has been determined based on the unutilized waste heat.
This project consists of several technologies described afterward, among those technologies, typical characteristic technologies are:
-
To compensate the endothermic reaction of hydrogen reduction by minimizing the carbon direct reduction using improvement of blast conditions. Although to decrease the carbon direct reduction is fundamental and essential approaches in the blast furnace operation, we pursue further innovative and multiple effects for this object.
-
For the application of COG for iron ore reduction, COG is reformed partially and applied to adequate region in the blast furnace. Effective reforming technology and application methods are innovative technology.
-
For sequestration of CO2, effective chemical absorbent, physical adsorbent and utilization system for unused heat in the steel works. Research and development of effective agents and heat-exchange facilities are innovative technology.
Technologies for Reducing Carbon Dioxide Emissions from the Blast Furnace
Figure 2 shows the concept of the intensified hydrogen reduction of iron ore. Conventional methods consist on the CO indirect reduction ratio (60 %), carbon direct reduction ratio (30 %), and hydrogen reduction ratio (10 %). On the other hand, in the case of intensified hydrogen reduction, it is necessary to ensure the heat balance by decreasing the carbon direct reduction, which is also an endothermic reaction. As shown in the image on the right half in Fig. 2, if a direct reduction ratio of 20 % and indirect reduction ratio of 60 % are obtained, a hydrogen reduction ratio of about 20 % is feasible from the view point of the heat balance. (These values are qualitative examples. For specific estimations, a mathematical simulation model will be used.)
Concept of the intensified hydrogen reduction of iron ore [7]
However, as described above, the decrease in carbon direct reduction implies an industrial process restriction in thermodynamic terms, and this is a challenge of intensified hydrogen reduction.
There are two possibilities for the application of COG in the blast furnace. The first is the method of injecting COG from the tuyere, which can utilize high temperatures in excess of 2000 °C. In this case, the injected gas spreads out over a wide area inside the furnace.
The second possibility is injection from the shaft, which is at a temperature of less than 1100 °C, where the direct carbon reaction is not favorable. This is the concept of shaft injection, even if the effect of shaft injection is limited to near the wall of blast furnace, and COG reforming is required.
COURSE50 verified the basic concept of intensified hydrogen reduction using the LKAB experimental blast furnace [8] to investigate and evaluate the potential for replacing coke and coal as reducing agents in the blast furnace with high H2 containing gas such as COG or RCOG (Reformed COG). The test operation started on April 16, 2012 and finished on May 11, 2012 (Table 2).
The test results are shown in Fig. 3. In both cases, direct reduction decreased compared to the base case, and hydrogen reduction increased.
Comparison of the distribution of the reduction step for COG and reformed COG injection [7]
Figure 4 shows the results of material and heat balance calculations using a mathematical simulation model considering the furnace gas flow to estimate the temperature distribution and hydrogen concentrations in the test blast furnace. The simulation model considers gas–liquid–solid phase chemical reactions and phase changes based on the mass, momentum, and energy conservation.
Temperature profiles calculated by a mathematical simulation model [7]
Figure 5 shows the results of COG and RCOG injected into the LKAB experimental blast furnace. The calculated values have been done by three-dimensional simulation model [9]. The high reaction rate of hydrogen in the COG injection from the tuyere and reformed COG injection from the shaft resulted in an increase in hydrogen reduction. As a result of this experiment, no differences between COG injection from the tuyere and reformed COG injection from shaft could be observed. The reason for this might be incorrect condition settings for the shaft injection.
Comparison of the carbon consumption rate between the observed and calculated rates [7]
As a result, the carbon consumption rate compared to the base case was confirmed to decrease by about 3 % for both COG and reformed COG (Fig. 5), and the result confirmed the direction of the COURSE50 project. The pathway to achieving the target is now under consideration through material and heat balance calculations using a mathematical simulation model. The main supporting technology is lowering carbon direct reduction.
Comparison of the COG injection method shows no significant difference.
According to the survey analysis results of the blast furnace (Fig. 6), following injection of reformed COG from the shaft furnace, the hydrogen concentration near the wall increased. The carbon consumption rates in the whole furnace show no noted difference, but both in-furnace gas reaction behaviors were significantly different.
Radial distribution of H2 concentration in the bosh gas measured by the upper shaft probe [7]
The characteristics of the coke required for the intensified hydrogen reduction steelmaking are also being investigated. In particular, we are trying to produce a high strength coke to maintain the gas permeability necessary for the intensified hydrogen reduction technology under reduced coke rates compared with conventional blast furnace operation. Figure 7 shows the procedure for producing high strength coke by applying a high performance caking additive called HPC.
HPC starts softening and melting at temperatures below 300 °C. Therefore, HPC packs the coal particles effectively by filling gaps in between the particles. As a result, it is possible to produce a high strength coke with HPC as shown in Fig. 8. The characteristic of HPC is the low sulfur content because HPC is not derived from oil, and it can be used in any quantitative until the desired performance is achieved.
Experimental results of coke strength enhancement using HPC [10]
The coke matrix connectivity index indicates the ratio of strongly connected matrix in the coke, and it is defined as the ratio of the largest coke-matrix connected by a thick wall of more than 15.2 μm to the whole coke-matrix within the cross-sectional surface of a coke specimen (e.g., φ20 mm) that is obtained by image analysis.
Figure 9 shows the effect of HPC, which improves the connecting Index and coke strength based on the same mechanism as the matrix control technology for coke.
Relation between coke-matrix connectivity index and coke strength [11]
Sequestration of CO2 Using Unused Waste Heat
Another project is developing technology to capture CO2 from blast furnace gas (BFG) through the chemical absorption and physical adsorption methods using unused waste heat in the steelworks (for example, from the sintering process, cooler exhaust gas, hot stove gas, steelmaking slag). The typical process flow for chemical absorption is shown in Figs. 10 and 11. The absorbent comes in contact with the feed gas in a counter-current absorber and selectively absorbs CO2. (2) CO2-rich absorbent is sent to the stripper and where CO2 is released by heating at about 120 °C. (3) The regenerated absorbent is cooled and sent back to the absorber to repeat the cycle.
Chemical absorption process [7]
We developed a chemical absorption method that can reduce the thermal energy consumption for the CO2 separation by half from 4GJ/t-CO2 to 2GJ/t-CO2 through a high performance absorbent and improvements to the chemical absorption process as shown in Fig. 12. Two pilot test plants were used to develop the process. The first one is CAT1, which has a capacity of 1 ton-CO2/day. It was used to evaluate the fundamental performance of the absorbent. The second one is CAT30, which has a capacity of 30 ton-CO2/day. It was used to extensively evaluate the absorbent selected following testing in CAT1. In addition to low energy consumption, the new absorbent has another unique and favorable feature. It easily releases CO2 at a lower temperature than that of the conventional regeneration process. This means that it may be possible to utilize low temperature waste heat at low cost.
Thermal energy consumption for CO2 capture [7]
Utilization of unused waste heats will be achieved through.
-
Utilization of unused waste heat whose condition did not match the economic potential for the power generation.
-
Utilization of unused waste heat whose technology has not been developed yet for example the use of BOF slag sensible heat.
For separating CO2 from BFG, we are also developing a physical adsorption method utilizing PSA technology. Figure 13 shows the concept of our PSA system. CO2 is separated in the first PSA unit by the CO2 adsorbent. Off-gas from the first PSA mainly consists of N2 and CO. This off-gas is introduced into the second PSA unit where CO is adsorbed by the adsorbent and separated from N2. Figure 14 shows a 3 tons/d pilot plant scale PSA facility.
Concept of the two-staged PSA for blast furnace gas [12]
Figure 15 shows the results of the general test operation using a bench scale plant called ASCOA-3, which had a capacity of 3 ton-CO2/day in STEP1. All of the target objectives were achieved during the test operation period. The maximum purity was 99.5 % with 3 tons of CO2 recovery.
Test operation results in the physical adsorption plant [10]
Energy such as steam and electricity is required for the above CO2 capture from blast furnace gas. If the energy is procured from external sources, CO2 will be emitted at the energy production sites. Therefore, in this project, the technology will be developed with unused waste heat that has not been utilized in the steelworks due to technological and/or economic difficulties.
One of the ideas that can be used for the chemical absorption method and physical adsorption method is described below. Unused waste heat from the steelworks can be converted into steam and used to regenerate the chemical absorbent or generate power for physical adsorption.
Power generation efficiency is a function of the temperature of the heat source as shown in Fig. 16.
Influence of heat source temperature on the required energy [7]
Therefore, the heat source temperature and quantity of the unused waste heat will determine the optimum configuration, and R&D is being promoted based on both conditions.
Pathway to the Target
Figures 17 and 18 provide an outline of the research and development schedule and the pathway to the mitigation of 30 % CO2 from the integrated steel plants. The effects of lowering CO2 emissions from the blast furnace are illustrated schematically in Fig. 18, where a simple application of COG is explored in LKAB experimental BF.
Schedule of research and development [7]
Figure 18 also shows the difference between effects of summation of each technology and the effect of total steel plant which is caused by the change of energy balances between before and after the introduction of this technology.
Conclusion
The COURSE50 project is based on two concepts. One is the reduction of iron ore by hydrogen as an alternative reduction agent, and the other is a combination of high efficiency CO2 separation technology utilizing unused energy.
Innovative technology development is being executed under the R&D schedule (Fig. 17), and currently, the integration of intensified hydrogen reduction and CO2 separation and recovery is being studied.
References
National Institute for Environmental Studies (2015) National greenhouse gas inventory report of JAPAN. National Institute for Environmental Studies, Tukuba
International comparison of energy efficiency in the steel industry (The Japan iron and steel federation), http://www.jisf.or.jp/en/activity/climate/documents/Reportofcommitmenttoalowcarbonsociety.pdf. Accessed 10 Sept 2013
ISIJ (eds) (2014) The 1st volume ironmaking and steelmaking. Handbook of iron and steel, 5th edn. Letter Press, Hiroshima pp 131–132
ISIJ (eds) (2014) The 1st volume ironmaking and steelmaking. Handbook of iron and steel, 5th edn. Letter Press, Hiroshima, pp 303–304
ISIJ (eds) (2014) The 1st volume ironmaking and steelmaking. Handbook of iron and steel, 5th edn. Letter Press, Hiroshima, pp 207–215
Miwa T, Okuda H (2010) CO2 ultimate reduction in steelmaking process by innovative technology for cool earth 50(COURSE50). J Jpn Inst Energy 89:28–35
Ueno H, Endo S, Tomomura S, Ishiwata N (2015) Outline of CO2 ultimate reduction in steelmaking process by innovative technology for cool earth 50(COURSE50 project). J Jpn Inst Energy 94:1277–1283
Watakabe S, Miyagawa K, Matsuzaki S, Inada T, Tomita Y, Saito K, Osame M, Sikström P, Ökvist LS, Wikstrom J (2013) Operation trial of hydrogenous gas injection of COURSE50 project at an experimental blast furnace. ISIJ Int 53:2065–2071
Takatani K, Inada T, Ujisawa Y (1999) Three-dimensional dynamic simulator for blast furnace. ISIJ Int 39:15–22
Watakabe S (2013) Current progress on COURSE50 project−recent results on H2 reduction including LKAB EBF’s experiments and CO2 capture. IEAGHG/IETS Iron and Steel Industry CCUS and Process Integration workshop, Tokyo, Japan
Sakimoto N, Takanohashi T, Sakai K, Shishido T, Yoshida T, Okuyama N (2015) Estimation of coke drum index from coke matrix connectivity. The 13th China-Japan Symposium on Coal and C1 Chemistry, Dunhuang, China
Saima H (2013) IEAGHG/IETS iron and steel industry CCUS and process integration workshop. Tokyo, Japan, pp 5–7
Acknowledgments
This study has been carried out as a national contract research project “development of technologies for an environmentally harmonized steelmaking process” under COURSE50 commissioned by the New Energy and Industrial Technology Development Organization (NEDO). The authors are grateful to the NEDO for their support.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was Sharif Jahanshahi.
Rights and permissions
About this article
Cite this article
Tonomura, S., Kikuchi, N., Ishiwata, N. et al. Concept and Current State of CO2 Ultimate Reduction in the Steelmaking Process (COURSE50) Aimed at Sustainability in the Japanese Steel Industry. J. Sustain. Metall. 2, 191–199 (2016). https://doi.org/10.1007/s40831-016-0066-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-016-0066-4