Abstract
Purpose of Review
The purpose of this review is to summarize the current status of in utero gene therapy and postnatal in vivo genome editing with an eye toward prenatal genome editing.
Recent Findings
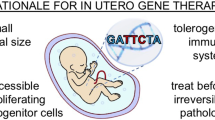
The rational for gene therapy and genome editing in utero is described, specifically the small size of the fetus, fetal immunologic immaturity, prenatal accessibility of stem and progenitor cells, and the ability to treat target diseases prior to birth. We review studies in normal and disease small and large animal models which demonstrate the feasibility and safety of in utero gene therapy using a variety of viral vectors. Postnatal in vivo genome editing with CRISPR-Cas9 in a number of murine disease models is discussed including the preference for nonhomologous end-joining compared to homology-directed repair in non-proliferating adult cells as a potential benefit to application of CRISPR-Cas9 genome editing to the fetus. Finally, the ethical challenges of human in utero gene therapy are discussed in the context of the EVERREST trial that is currently being designed.
Summary
In utero gene therapy and genome editing is a developing field with great potential to treat congenital monogenic diseases. More research in small and large animal models is required before clinical translation can occur.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Naldini L. Gene therapy returns to centre stage. Nature. 2015;526(7573):351–60. https://doi.org/10.1038/nature15818.
Maeder ML, Gersbach CA. Genome-editing technologies for gene and cell therapy. Mol Ther. 2016;24(3):430–46. https://doi.org/10.1038/mt.2016.10.
Dilworth MR, Kusinski LC, Baker BC, Renshall LJ, Greenwood SL, Sibley CP, et al. Defining fetal growth restriction in mice: a standardized and clinically relevant approach. Placenta. 2011;32(11):914–6. https://doi.org/10.1016/j.placenta.2011.08.007.
Owen RD. Immunogentic consequences of vascular anastomoses between bovine twins. Science. 1945;102(2651):400–1. https://doi.org/10.1126/science.102.2651.400.
Witt R, MacKenzie TC, Peranteau WH. Fetal stem cell and gene therapy. Semin Fetal Neonatal Med. 2017;22(6):410–4. https://doi.org/10.1016/j.siny.2017.05.003.
Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–6.
Simonsen M. The acquired immunity concept in kidney homotransplantation. Ann N Y Acad Sci. 1955;59(3):448–52. https://doi.org/10.1111/j.1749-6632.1955.tb45959.x.
Calcedo R, Griesenbach U, Dorgan DJ, Soussi S, Boyd AC, Davies JC, et al. Self-reactive CFTR T cells in humans: implications for gene therapy. Hum Gene Ther Clin Dev. 2013;24(3):108–15. https://doi.org/10.1089/humc.2012.249.
Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13(4):419–22. https://doi.org/10.1038/nm1549.
Calcedo R, Morizono H, Wang L, McCarter R, He J, Jones D, et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol. 2011;18(9):1586–8. https://doi.org/10.1128/CVI.05107-11.
Wang D, Mou H, Li S, Li Y, Hough S, Tran K, et al. Adenovirus-mediated somatic genome editing of Pten by CRISPR/Cas9 in mouse liver in spite of Cas9-specific immune responses. Hum Gene Ther. 2015;26(7):432–42. https://doi.org/10.1089/hum.2015.087.
Sabatino DE, Mackenzie TC, Peranteau W, Edmonson S, Campagnoli C, Liu YL, et al. Persistent expression of hF.IX after tolerance induction by in utero or neonatal administration of AAV-1-F.IX in hemophilia B mice. Mol Ther. 2007;15(9):1677–85. https://doi.org/10.1038/sj.mt.6300219.
Davey MG, Riley JS, Andrews A, Tyminski A, Limberis M, Pogoriler JE, et al. Induction of immune tolerance to foreign protein via adeno-associated viral vector gene transfer in mid-gestation fetal sheep. PLoS One. 2017;12(1):e0171132. https://doi.org/10.1371/journal.pone.0171132.
Colletti E, Lindstedt S, Park PJ, Almeida-Porada G, Porada CD. Early fetal gene delivery utilizes both central and peripheral mechanisms of tolerance induction. Exp Hematol. 2008;36(7):816–22. https://doi.org/10.1016/j.exphem.2008.02.007.
Tran ND, Porada CD, Almeida-Porada G, Glimp HA, Anderson WF, Zanjani ED. Induction of stable prenatal tolerance to beta-galactosidase by in utero gene transfer into preimmune sheep fetuses. Blood. 2001;97(11):3417–23. https://doi.org/10.1182/blood.V97.11.3417.
Meertens L, Zhao Y, Rosic-Kablar S, Li L, Chan K, Dobson H, et al. In utero injection of alpha-L-iduronidase-carrying retrovirus in canine mucopolysaccharidosis type I: infection of multiple tissues and neonatal gene expression. Hum Gene Ther. 2002;13(15):1809–20. https://doi.org/10.1089/104303402760372918.
Garrett DJ, Larson JE, Dunn D, Marrero L, Cohen JC. In utero recombinant adeno-associated virus gene transfer in mice, rats, and primates. BMC Biotechnol. 2003;3(1):16. https://doi.org/10.1186/1472-6750-3-16.
Yin H, Kauffman KJ, Anderson DG. Delivery technologies for genome editing. Nat Rev Drug Discov. 2017;16(6):387–99. https://doi.org/10.1038/nrd.2016.280.
Potter H, Heller R. Transfection by electroporation. Curr Protoc Immunol. 2017;117:10.5.1–9. https://doi.org/10.1002/cpim.24.
Han X, Liu Z, Jo MC, Zhang K, Li Y, Zeng Z, et al. CRISPR-Cas9 delivery to hard-to-transfect cells via membrane deformation. Sci Adv. 2015;1(7):e1500454. https://doi.org/10.1126/sciadv.1500454.
Wu Y, Liang D, Wang Y, Bai M, Tang W, Bao S, et al. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell. 2013;13(6):659–62. https://doi.org/10.1016/j.stem.2013.10.016.
•• Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548(7668):413–9. https://doi.org/10.1038/nature23305. This is the first described in vitro CRISPR-Cas9 homology-directed repair of human embryonic cells with correction in all cells.
Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol. 2015;33(1):73–80. https://doi.org/10.1038/nbt.3081.
Ahi YS, Bangari DS, Mittal SK. Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther. 2011;11(4):307–20. https://doi.org/10.2174/156652311796150372.
Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073–80. https://doi.org/10.1038/mt.2008.76.
Lisowski L, Tay SS, Alexander IE. Adeno-associated virus serotypes for gene therapeutics. Curr Opin Pharmacol. 2015;24:59–67. https://doi.org/10.1016/j.coph.2015.07.006.
Majowicz A, Salas D, Zabaleta N, Rodriguez-Garcia E, Gonzalez-Aseguinolaza G, Petry H, et al. Successful repeated hepatic gene delivery in mice and non-human primates achieved by sequential administration of AAV5ch and AAV1. Mol Ther. 2017;25(8):1831–42. https://doi.org/10.1016/j.ymthe.2017.05.003.
Mattar CN, Nathwani AC, Waddington SN, Dighe N, Kaeppel C, Nowrouzi A, et al. Stable human FIX expression after 0.9G intrauterine gene transfer of self-complementary adeno-associated viral vector 5 and 8 in macaques. Mol Ther. 2011;19(11):1950–60. https://doi.org/10.1038/mt.2011.107.
Sugano H, Matsumoto T, Miyake K, Watanabe A, Iijima O, Migita M, et al. Successful gene therapy in utero for lethal murine hypophosphatasia. Hum Gene Ther. 2012;23(4):399–406. https://doi.org/10.1089/hum.2011.148.
Dejneka NS, Surace EM, Aleman TS, Cideciyan AV, Lyubarsky A, Savchenko A, et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther. 2004;9(2):182–8. https://doi.org/10.1016/j.ymthe.2003.11.013.
Endo M, Henriques-Coelho T, Zoltick PW, Stitelman DH, Peranteau WH, Radu A, et al. The developmental stage determines the distribution and duration of gene expression after early intra-amniotic gene transfer using lentiviral vectors. Gene Ther. 2010;17(1):61–71. https://doi.org/10.1038/gt.2009.115.
Joyeux L, Danzer E, Limberis MP, Zoltick PW, Radu A, Flake AW, et al. In utero lung gene transfer using adeno-associated viral and lentiviral vectors in mice. Hum Gene Ther Methods. 2014;25(3):197–205. https://doi.org/10.1089/hgtb.2013.143.
Stitelman DH, Brazelton T, Bora A, Traas J, Merianos D, Limberis M, et al. Developmental stage determines efficiency of gene transfer to muscle satellite cells by in utero delivery of adeno-associated virus vector serotype 2/9. Mol Ther Methods Clin Dev. 2014;1:14040. https://doi.org/10.1038/mtm.2014.40.
• Mattar CNZ, Gil-Farina I, Rosales C, Johana N, Tan YYW, McIntosh J, et al. In utero transfer of adeno-associated viral vectors produces long-term factor IX levels in a cynomolgus macaque model. Mol Ther. 2017;25(8):1843–53. https://doi.org/10.1016/j.ymthe.2017.04.003. In utero primate gene therapy for hemophilia results in long term therapeutic human factor IX gene expression.
Shen JS, Meng XL, Yokoo T, Sakurai K, Watabe K, Ohashi T, et al. Widespread and highly persistent gene transfer to the CNS by retrovirus vector in utero: implication for gene therapy to Krabbe disease. J Gene Med. 2005;7(5):540–51. https://doi.org/10.1002/jgm.719.
Reay DP, Bilbao R, Koppanati BM, Cai L, O'Day TL, Jiang Z, et al. Full-length dystrophin gene transfer to the mdx mouse in utero. Gene Ther. 2008;15(7):531–6. https://doi.org/10.1038/gt.2008.8.
Abi-Nader KN, David AL. Fetal muscle gene therapy/gene delivery in large animals. Methods Mol Biol. 2011;709:239–56. https://doi.org/10.1007/978-1-61737-982-6_15.
Lin S, Staahl BT, Alla RK, Doudna JA. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. elife. 2014;3:e04766. https://doi.org/10.7554/eLife.04766.
Roybal JL, Endo M, Radu A, Gray L, Todorow CA, Zoltick PW, et al. Early gestational gene transfer with targeted ATP7B expression in the liver improves phenotype in a murine model of Wilson’s disease. Gene Ther. 2012;19(11):1085–94. https://doi.org/10.1038/gt.2011.186.
Han XD, Lin C, Chang J, Sadelain M, Kan YW. Fetal gene therapy of alpha-thalassemia in a mouse model. Proc Natl Acad Sci U S A. 2007;104(21):9007–11. https://doi.org/10.1073/pnas.0702457104.
Endo M, Zoltick PW, Radu A, Jiang Q, Qiujie J, Matsui C, et al. Early intra-amniotic gene transfer using lentiviral vector improves skin blistering phenotype in a murine model of Herlitz junctional epidermolysis bullosa. Gene Ther. 2012;19(5):561–9. https://doi.org/10.1038/gt.2011.135.
Waddington SN, Buckley SM, Nivsarkar M, Jezzard S, Schneider H, Dahse T, et al. In utero gene transfer of human factor IX to fetal mice can induce postnatal tolerance of the exogenous clotting factor. Blood. 2003;101(4):1359–66. https://doi.org/10.1182/blood-2002-03-0779.
David A, Cook T, Waddington S, Peebles D, Nivsarkar M, Knapton H, et al. Ultrasound-guided percutaneous delivery of adenoviral vectors encoding the beta-galactosidase and human factor IX genes to early gestation fetal sheep in utero. Hum Gene Ther. 2003;14(4):353–64. https://doi.org/10.1089/104303403321208952.
Keswani SG, Balaji S, Katz AB, King A, Omar K, Habli M, et al. Intraplacental gene therapy with Ad-IGF-1 corrects naturally occurring rabbit model of intrauterine growth restriction. Hum Gene Ther. 2015;26(3):172–82. https://doi.org/10.1089/hum.2014.065.
Carr DJ, Wallace JM, Aitken RP, Milne JS, Martin JF, Zachary IC, et al. Peri- and postnatal effects of prenatal adenoviral VEGF gene therapy in growth-restricted sheep. Biol Reprod. 2016;94(6):142. https://doi.org/10.1095/biolreprod.115.133744.
Carr DJ, Wallace JM, Aitken RP, Milne JS, Mehta V, Martin JF, et al. Uteroplacental adenovirus vascular endothelial growth factor gene therapy increases fetal growth velocity in growth-restricted sheep pregnancies. Hum Gene Ther. 2014;25(4):375–84. https://doi.org/10.1089/hum.2013.214.
• Sheppard M, Spencer RN, Ashcroft R, David AL, Consortium E. Ethics and social acceptability of a proposed clinical trial using maternal gene therapy to treat severe early-onset fetal growth restriction. Ultrasound Obstet Gynecol. 2016;47(4):484–91. https://doi.org/10.1002/uog.15880. This paper explores ethical considerations for the implementation of the EVERREST trial.
Krishnan T, David AL. Placenta-directed gene therapy for fetal growth restriction. Semin Fetal Neonatal Med. 2017;22(6):415–22. https://doi.org/10.1016/j.siny.2017.04.005.
Lieber MR, Gu J, Lu H, Shimazaki N, Tsai AG. Nonhomologous DNA end joining (NHEJ) and chromosomal translocations in humans. Subcell Biochem. 2010;50:279–96. https://doi.org/10.1007/978-90-481-3471-7_14.
Heyer WD, Ehmsen KT, Liu J. Regulation of homologous recombination in eukaryotes. Annu Rev Genet. 2010;44(1):113–39. https://doi.org/10.1146/annurev-genet-051710-150955.
Rosen LE, Morrison HA, Masri S, Brown MJ, Springstubb B, Sussman D, et al. Homing endonuclease I-CreI derivatives with novel DNA target specificities. Nucleic Acids Res. 2006;34(17):4791–800. https://doi.org/10.1093/nar/gkl645.
Grizot S, Smith J, Daboussi F, Prieto J, Redondo P, Merino N, et al. Efficient targeting of a SCID gene by an engineered single-chain homing endonuclease. Nucleic Acids Res. 2009;37(16):5405–19. https://doi.org/10.1093/nar/gkp548.
Arnould S, Perez C, Cabaniols JP, Smith J, Gouble A, Grizot S, et al. Engineered I-CreI derivatives cleaving sequences from the human XPC gene can induce highly efficient gene correction in mammalian cells. J Mol Biol. 2007;371(1):49–65. https://doi.org/10.1016/j.jmb.2007.04.079.
Wang J, Exline CM, DeClercq JJ, Llewellyn GN, Hayward SB, Li PW, et al. Homology-driven genome editing in hematopoietic stem and progenitor cells using ZFN mRNA and AAV6 donors. Nat Biotechnol. 2015;33(12):1256–63. https://doi.org/10.1038/nbt.3408.
Modares M, Shariati L, Hejazi Z, Shahbazi M, Tabatabaiefar MA, Khanahmad H. Inducing indel mutation in the SOX6 gene by zinc finger nuclease for gamma reactivation: an approach towards gene therapy of Beta thalassemia. J Cell Biochem. 2017; https://doi.org/10.1002/jcb.26412.
Sorek R, Kunin V, Hugenholtz P. CRISPR--a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat Rev Microbiol. 2008;6(3):181–6. https://doi.org/10.1038/nrmicro1793.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. https://doi.org/10.1126/science.1225829.
Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. GRNA-programmed genome editing in human cells. elife. 2013;2:e00471. https://doi.org/10.7554/eLife.00471.
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339(6121):823–6. https://doi.org/10.1126/science.1232033.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–23. https://doi.org/10.1126/science.1231143.
Kemaladewi DU, Maino E, Hyatt E, et al. Correction of a splicing defect in a mouse model of congenital muscular dystrophy type 1A using a homology-directed-repair-independent mechanism. Nat Med. 2017; https://doi.org/10.1038/nm4367.
Tabebordbar M, Zhu K, Cheng JKW, Chew WL, Widrick JJ, Yan WX, et al. In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science. 2016;351(6271):407–11. https://doi.org/10.1126/science.aad5177.
Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science. 2016;351(6271):403–7. https://doi.org/10.1126/science.aad5143.
Ouellet DL, Cherif K, Rousseau J, Tremblay JP. Deletion of the GAA repeats from the human frataxin gene using the CRISPR-Cas9 system in YG8R-derived cells and mouse models of Friedreich ataxia. Gene Ther. 2017;24(5):265–74. https://doi.org/10.1038/gt.2016.89.
• Yin H, Xue W, Chen S, Bogorad RL, Benedetti E, Grompe M, et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol. 2014;32(6):551–3. https://doi.org/10.1038/nbt.2884. This is one of the first in vivo studies using CRISPR-Cas9 to demonstrate disease model correction postnatally in a murine model of hereditary tyrosinemia, an otherwise fatal disease.
Ah Mew N, Krivitzky L, McCarter R, Batshaw M, Tuchman M. Network UCDCotRDCR. Clinical outcomes of neonatal onset proximal versus distal urea cycle disorders do not differ. J Pediatr. 2013;162(2):324–9.e1. https://doi.org/10.1016/j.jpeds.2012.06.065.
Hodges PE, Rosenberg LE. The spfash mouse: a missense mutation in the ornithine transcarbamylase gene also causes aberrant mRNA splicing. Proc Natl Acad Sci U S A. 1989;86(11):4142–6. https://doi.org/10.1073/pnas.86.11.4142.
Yang Y, Wang L, Bell P, McMenamin D, He Z, White J, et al. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat Biotechnol. 2016;34(3):334–8. https://doi.org/10.1038/nbt.3469.
Bennett J. Taking stock of retinal gene therapy: looking back and moving forward. Mol Ther. 2017;25(5):1076–94. https://doi.org/10.1016/j.ymthe.2017.03.008.
George LA, Fogarty PF. Gene therapy for hemophilia: past, present and future. Semin Hematol. 2016;53(1):46–54. https://doi.org/10.1053/j.seminhematol.2015.10.002.
Porada CD, Park PJ, Tellez J, Ozturk F, Glimp HA, Almeida-Porada G, et al. Male germ-line cells are at risk following direct-injection retroviral-mediated gene transfer in utero. Mol Ther. 2005;12(4):754–62. https://doi.org/10.1016/j.ymthe.2005.05.011.
Park PJ, Colletti E, Ozturk F, Wood JA, Tellez J, Almeida-Porada G, et al. Factors determining the risk of inadvertent retroviral transduction of male germ cells after in utero gene transfer in sheep. Hum Gene Ther. 2009;20(3):201–15. https://doi.org/10.1089/hum.2007.120.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Heather A. Hartman, Avery C. Rossidis, and William H. Peranteau declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Prenatal Therapies
Rights and permissions
About this article
Cite this article
Hartman, H.A., Rossidis, A.C. & Peranteau, W.H. In Utero Gene Therapy and Genome Editing. Curr Stem Cell Rep 4, 52–60 (2018). https://doi.org/10.1007/s40778-018-0117-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-018-0117-9