Abstract
Introduction
There are few biomarkers correlated with psoriatic arthritis (PsA). We aimed to explore the clinical value of calprotectin (CLP) in PsA in disease activity and treatment targets.
Methods
Serum CLP was detected by enzyme-linked immunosorbent assay (ELISA) in 71 patients with PsA, 55 patients with psoriasis (PsO), and 10 healthy controls. The association of serum CLP with disease activity index at baseline and follow-up was analyzed. Cox regression and receiver operating characteristic (ROC) analysis were used to evaluate the potential of CLP for predicting the achievement of treatment targets, including low disease activity (LDA), remission, and minimal disease activity (MDA).
Results
Serum CLP levels (μg/ml) were significantly increased in patients with PsA/PsO compared with healthy controls (p < 0.001). Serum CLP levels were positively associated with psoriasis area and severity index (PASI), disease activity in psoriatic arthritis (DAPSA), and its components [including tender joint count (TJC), swollen joint count (SJC), patient’s global assessment (PGA), and visual analog scale (VAS)-pain, r 0.290–0.601, all p value < 0.05]. After 1-year follow-up, the number of patients with PsA in remission and MDA increased [17 (23.9%) versus 47 (66.1%) and 21 (29.5%) versus 52 (73.2%) respectively, all p value < 0.001]. Cox regression and Kaplan–Meier survival analysis indicated that patients with lower CLP obtain LDA, MDA, and remission earlier, including remission and MDA within a year (all p-value < 0.05). ROC analysis showed the ability of serum at baseline to predict the achievement of the treatment target in 3 months [area under the curve (AUC) 0.663–0.691, all p-values < 0.05].
Conclusions
Serum CLP level was correlated with disease activity in PsA. It also possessed the ability to predict the achievement of the therapeutic target. These features of CLP would make it a useful tool in clinical work.
Plain Language Summary
Psoriatic disease is a group of heterogeneous inflammatory conditions, mainly affecting patients’ skin and joints. Among the few biomarkers associated with inflammation in psoriatic disease, serum calprotectin may be a promising one. Therefore, we conducted a longitudinal study to explore the clinical significance of calprotectin in patients with psoriatic arthritis at Peking University First Hospital. We found that the level of serum calprotectin positively correlated with disease activity parameters and scores in psoriatic arthritis. It also possessed the ability to predict the achievement of treatment targets, including remission and minimal disease activity. On the basis of the findings of our study, we reckon that CLP may be a useful tool in clinical work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Previous studies suggested serum calprotectin (CLP) as an inflammatory biomarker with some doubts regarding its effectiveness in diagnosing and monitoring psoriatic arthritis (PsA). |
What was learned from this study? |
Serum CLP correlated with disease activity in PsA and decreased after treatment. |
Lower serum CLP levels indicated sooner achievement of treatment targets in patients with PsA. |
Serum CLP could be a useful tool in monitoring treatment response and predicting therapeutic target achievement in PsA. |
Introduction
Psoriasis (PsO) is a chronic inflammatory disease affecting the skin that may exhibit a variety of clinical manifestations. In China, it has been shown to affect approximately 0.14% of the total population [1]. About 20–25% of patients with psoriatic disease will develop psoriatic arthritis [2], with presentation of dactylitis, enthesitis, synovitis, and so on. In addition to skin and joint involvement, some comorbidities such as cardiovascular disease, metabolic syndrome, malignancies, and mental disorders occur with the psoriatic disease [3, 4]. The peak age range for the onset of psoriatic disease is 30–39 years [5]. In addition to the suffering this disease inflicts on patients, it also has many negative effects on society, such as financial burdens that result from controlling and treating these chronic diseases. Evaluation of disease in psoriatic disease usually depends on compound clinical indices [6, 7].
The recent European Alliance of Associations for Rheumatology (EULAR) recommendation for the management of psoriatic arthritis indicates that the goal is to reach low disease activity (LDA) or remission [8]. However, the frequency of LDA/remission was relatively low in previously reported studies [9].
In recent years, many studies have focused on finding a valid biomarker with high sensitivity and specificity for diagnosing and monitoring psoriatic disease. Among these studies, many have reported promising results for calprotectin (CLP). CLP is a heterodimeric complex formed by two calcium-binding proteins that belong to the S-100 protein family, S100A8 and S100A9, with pro-inflammatory properties [10]. There are still some doubts about the effectiveness of diagnosing and monitoring psoriatic arthritis, especially with regard to predicting the treatment targets. In the present study, we evaluated the serum CLP concentration and explored its association with the disease as well as therapeutic response in patients with PsA.
Methods
Study Design and Patients
This was a longitudinal study. Patients with PsA/PsO were recruited from the rheumatology and immunology department of Peking First University First Hospital from January 2019 to March 2021. Patients with PsA were followed up for 12 months. Patients with PsA fulfilled the Classification Criteria for PsA (CASPAR) [11] with ages above 18 years. Patients with other autoimmune diseases or nonpsoriatic inflammatory arthritis were excluded. The clinical and laboratory profiles, including tender joint count (TJC), swollen joint count (SJC), patient’s global assessment (PGA), VAS-pain (0–100), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), were recorded at baseline, 3 months, 6 months, and 1 year. Psoriasis area and severity index (PASI) was calculated for patients with PsA/PsO. Medications used at baseline were also collected.
The study was approved by the institutional Research Ethics Committee, and all patients gave informed consent for data collection of their medical records.
Evaluation of Clinical Disease Activity and Treatment Response in Patients with PsA
Disease activity in psoriatic arthritis (DAPSA) score was used to describe the activity of PsA [12]. DAPSA score was calculated by summing up the following variables: SJC, TJC, PGA, VAS-pain, and CRP. Clinical DAPSA (cDAPSA) was defined as DAPSA without CRP. The following cutoff values were proposed: ≤ 4 for remission (REM), > 4 and ≤ 14 for low disease activity (LDA), > 14 and ≤ 28 for moderate disease activity, and > 28 for high disease activity (HDA). Minimal disease activity (MDA) was defined as meeting five of the following criteria: TJC ≤ 1, SJC ≤ 1, PASI ≤ 1 or body surface area ≤ 3, VAS-pain ≤ 15, PGA ≤ 20, health assessment questionnaire ≤ 0.5, and tender entheseal points ≤ 1 [13].
Whether the recruited patients with PsA achieved LDA, remission, or MDA was the primary outcome in the study. The time to achieve these treatment targets above was also recorded. The American College of Rheumatology (ACR) responses were considered the secondary outcomes. ACR 20/50/70 was defined by the following three conditions: at least 20%/50%/70% improvement of TJC and SJC and at least 20%/50%/70% improvement in three of five additional domains (PGA, evaluators' global assessment, VAS-pain, health assessment questionnaire, and ESR/CRP) [14].
ELISA Analysis of Serum CLP
Serum samples obtained from patients with PsA/PsO and healthy controls were stored at −80 °C until analysis. The concentration of serum CLP (S100A8/A9 heterodimer) was measured using an ELISA kit (Human S100A8/S100A9 Heterodimer Quantikine ELISA Kit, R&D Systems) by the manufacturer’s protocol. Briefly, 50 μl of diluted 1:200 serum sample in duplicate wells was incubated for 2 h. After three washes, 200 μl conjugate was added to each well for another 2 h incubation. After three washes and the addition of enzyme substrate, an optical density reader was subsequently used to measure wavelength of 450 nm, with 540 nm used as a wavelength correction. The results of CLP were expressed as median (IQR), in μg/mL.
Statistical Analysis
Data were presented as mean (SD), median (IQR), or proportion (%) as appropriate. Comparisons of continuous variables with normal distribution such as age were performed using Student’s t-test. Other continuous variables not fulfilling normal distribution were evaluated by Mann–Whitney U test. Categorical variables were reported as frequencies and analyzed by chi-squared test. Pearson’s correlation was used to analyze the linear association. The predictive power of the model was accessed by measuring the area under the receiver operating characteristic (ROC) curve. The cutoff value was determined by the ROC with the highest Youden index. Results of Cox regression analysis were presented as hazard ratio (HR) and 95% confidence intervals (CIs). Kaplan–Meier curves were used to visualize the change of reaching the therapeutic target during the 1-year follow-up. Statistical significance was analyzed by SPSS version 26.0 software and GraphPad Prism V 8.00.
Results
Demographic and Clinical Characteristics of Patients with PsA at Baseline
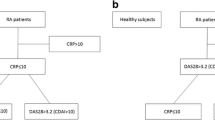
A total of 71 patients with PsA, 55 patients with PsO, and 50 healthy controls (HCs) were recruited at baseline. All patients and HCs were of Han nationality. Their age [50.2 (14.3) versus 47.9 (12.6) versus 48.1 (6.3) years] and male gender proportion (61.9% versus 49.0% versus 54.0%) were comparable among all three groups (p > 0.05 for all). The duration of skin lesion at first visit [median (IQR) 15.0 (4.0, 22.2) versus 19.5 (9.2, 26.9) years], ESR [median (IQR) 12.0 (4.0, 25.0) versus 10.0 (6.0, 17.0) mm/h], CRP [median (IQR) 3.2 (1.4, 11.3) versus 4.0 (1.9, 6.1) mg/L], and PASI [median (IQR) 2.6 (0.3, 7.1) versus 2.8 (0.8, 5.1)] were similar between patients with PsA and those with PsO (Supplementary Table S1).
At baseline, the median DAPSA score was 12.9, with proportions of REM, LDA, moderate disease, and HDA being 17 (23.9%), 21 (29.5%), 15 (21.1%), and 18 (25.4%) for patients with PsA, respectively. Twenty-one (29.5%) patients with PsA were considered MDA at baseline. All patients with PsA were initiated with conventional synthetic disease-modified antirheumatic drugs (csDMARDs). Among them, 61 patients were prescribed methotrexate and 26 patients were prescribed biologics or Janus kinase inhibitor (JAKi) (Table 1).
Serum CLP Concentration and Its Correlation with Disease Activity in PsA/PsO at Baseline
Higher serum CLP was observed in patients with PsA/PsO than in healthy controls [median (IQR) 3.816 (2.368, 7.591) versus 0.707 (0.557, 0.836) μg/ml; 1.854 (1.123, 3.355) versus 0.707 (0.557, 0.836) μg/ml, p < 0.001 for both)]. The level of serum CLP in patients with PsA was even higher than in patients with PsO [median (IQR) 3.816 (2.368, 7.591) versus 1.854 (1.123, 3.355) μg/ml, p < 0.001] (Supplementary Table S1).
Patients with PsA with moderate or high disease activity (n = 33) exhibited higher concentrations of serum CLP than patients in LDA or remission (n = 38) [median (IQR) 6.139 (3.484, 9.500) versus 2.975 (2.201, 4.815), p < 0.001]. Disease may be more active in patients with PsA with serum CLP above 5.55 μg/ml (AUC 0.703, 95% CI 0.583–0.806, p = 0.002). In addition, median linear correlations between serum CLP concentrations and several indices (ESR, CRP, TJC, SJC, PGA, VAS-pain, and DAPSA score, r 0.301–0.601, all p value < 0.05) for arthritis activity were observed. Pearson’s coefficients of CLP with disease activity were higher than those with ESR or CRP (Supplementary Table S4). The linear correlation of PASI with CLP seems worse than the correlation with arthritis, although it was statistically significant in patients with PsA, and the linear correlation with serum CLP seemed to be strengthened after combining DAPSA and PASI (r = 0.679, p < 0.001) (Table 2). Serum CLP level was more closely correlated with PASI in patients with PsO (r = 0.663, p < 0.001).
Changes in Serum CLP after Treatment
During follow-up, a downtrend of disease activity was shown in our cohort (Table 1). The concentration of serum CLP was also decreased along with the disease activity [median (IQR) 3.816 (2.368, 7.591) versus 2.052 (1.222, 4.311) versus 1.681 (1.191, 2.577) versus 1.655 (1.438, 2.442), p < 0.001]. (Fig. 1) At month 3, month 6, and month 12, 50, 34, and 13 serum samples of patients with PsA were collected, respectively.
The decrease of serum CLP (ΔCLP) at month 3 was correlated with decreasing disease activity (r 0.323–0.570, all p value < 0.05) (Table 2). In 27 patients with PsA with serum samples at baseline, month 3, and month 6, we observed a decrease in serum CLP along with improvement of disease activity (Supplementary Table S2). A similar trend of CLP was also observed in 13 patients with PsA with serum samples of all follow-up points (baseline, month 3, month 6, and month 12) (Supplementary Table S3).
Predictive Values of Serum CLP for Achieving Therapeutic Targets
Cox regression adjusted for patients’ age, gender, arthritis duration, clinical manifestations (axial disease, psoriatic nail, uveitis) history of smoking, and initiation of biologics or JAKi showed that patients with higher serum CLP, instead of ESR or CRP, tended to take longer to obtain remission (HR 0.870, 95% CI 0.761–0.996, p = 0.043) or minimal disease activity (HR 0.874, 95% CI 0.778–0.982, p = 0.024) within a year. This trend was also observed in the probability of obtaining LDA, although no statistical significance was found (Table 3). Kaplan–Meier survival curves indicated that patients with a serum CLP concentration below 5.55 μg/ml at baseline will obtain treatment targets, including LDA, remission, and MDA, sooner than patients with higher CLP concentration (all p value < 0.05) (Table 4 and Fig. 2). We also used ROC curves to analyze the ability of baseline serum CLP to predict target achievement in 3 months. Although statistically significant, the AUCs may be small to validate our point of view (AUCs 0.663, 0.691, and 0.689 for LDA, remission, and MDA in 3 months, respectively, all p-value < 0.05) (Tables 5, 6 and Fig. 3).
ROC curves of baseline and reduction of serum CLP. (A)Identifying median to high disease activity, predicting therapeutic target (B LDA; C remission; D minimal disease activity) at 3 months and treatment response (E ACR50 in month 6; F ACR50 in month 12; G ACR70 in month 12). b-CLP/ESR/CRP calprotectin/ESR/CRP at baseline, ΔCLP/ESR/CRP reductions in calprotectin/ESR/CRP at month 3
Finally, ROC analysis revealed the ability of ΔCLP to predict treatment response. ΔCLP above 1.76 indicated a greater chance to obtain ACR50 in month 6 and 1 year (AUC 0.74–0.8, all p value < 0.001). When ΔCLP dropped more than 3.07 μg/ml, patients were more likely to obtain ACR70 in 1 year (AUC 0.794, p = 0.002).
Discussion
In this study, we analyzed the value of serum CLP as an inflammatory biomarker in PsA. We found that serum CLP positively correlated with disease activity, including both arthritis and skin lesion in PsA. We also observed a downtrend of serum CLP along with an improvement of PsA.
As a promising inflammatory biomarker, serum CLP has been found useful in monitoring disease activity and treatment response in rheumatoid arthritis and ankylosing spondylitis [15,16,17]. In PsO, serum CLP was related to the severity of skin lesions [18, 19]. With regard to PsA, the conclusion of the correlation varies. Aochi et al. observed that serum CLP levels were more closely related to joint manifestation instead of skin lesion [20], similarly to our findings. However, Jarlborg et al. did not find a correlation between serum CLP and DAPSA score; instead, skin lesions were more frequently reported in patients with PsA with higher serum CLP [21].
While the causes of the different conclusions regarding CLP and disease activity in PsA are still not clear, it is reasonable to assume that CLP could be involved in the pathogenesis of both arthritis and skin lesions in PsA. In our PsA cohort, high levels of serum CLP were observed in a few patients with relatively severe skin lesions and mild arthritis. The highest linear correlation with CLP was shown in the sum of both DAPSA and PASI scores. Therefore, we reckon that serum CLP levels were more closely related to systemic inflammation rather than single involvement of joint or skin.
Circulating CLP comes mainly from neutrophils and monocytes [22]. Many cytokines could regulate the expression of CLP, including tumor necrosis factor-α (TNFα), interferon-γ, and IL-10 [23]. We reckon that the IL-17/IL-23 axis may contribute to the overexpression of CLP. According to Bai et al., IL-17 stimulated neutrophils to release CLP [24]. In an animal experiment, IL-23 antibodies decreased the expression of transcript levels of S100 family members [25]. However, our hypothesis requires further exploration since the detailed mechanism of the regulation of circulating CLP exerted by the IL-17/23 axis has not been elucidated yet.
By activating the immunity pathway sensed by receptors of advanced glycation end products (RAGE) and Toll-like receptor-4 (TLR4), CLP participated in the progression of inflammatory [26]. Theoretically, by activating TLR4 on fibroblast-like synoviocytes, CLP could induce articular inflammation [23]. In addition, CLP was also involved in the inflammation process of enthesitis and psoriatic skin lesions [27, 28], which were the clinical features of PsA instead of rheumatoid arthritis (RA).
IL-6 is not the most important inflammatory mediator in PsA. Normal serum CRP was detected in about half of patients with PsA with active disease [29]. ESR is affected by many factors, including anemia and hypofibrinogenemia. These may undercut the accuracy of the traditional acute phase reactants mentioned above. However, we did not think that serum CLP could replace physical examination or musculoskeletal ultrasound, which could evaluate the severity of focal inflammation in PsA. Moreover, under some circumstances, the usage of serum CLP may be limited. For example, the detection of serum CLP in patients with neutrocytopenia may be inaccurate to assess inflammation since neutrophils are a major source of CLP. In addition, coagulants such as EDTA could harm the stability of CLP, which should be avoided when sampling [30].
Finally, in the present study we assessed the potential of CLP in monitoring and predicting treatment response. Few studies have focused on the changes in serum CLP in patients with PsA after receiving treatment. A study by Sokolova et al. observed the downtrend of serum CLP in several patients with PsA after receiving biologics [7], with results similar to those of the present study. In addition, we observed that patients with relatively low levels of serum CLP reached treatment targets sooner, including LDA, remission, and minimal disease activity. In a meta-analysis including 81 trials, less than half reported the status of remission or LDA in patients with PsA, and these patients account for only about one-third of the whole PsA population [9]. However the treatment target was mentioned in the EULAR recommendation [8]. Previous research on new biomarkers has focused on monitoring treatment response or predicting treatment outcomes. Recently, only a few studies have focused on serum biomarkers predicting treatment outcomes. To our knowledge, no previous research has focused on the predictive value of serum CLP in patients with PsA with regard to obtaining treatment targets. Our study illustrated the potential of serum CLP in PsA as an inflammatory biomarker by revealing its ability to monitor disease and predict treatment targets. These findings indicate that serum CLP may be useful for rheumatologists when making personal therapeutic plans for patients with PsA.
Our study has its limitations. First, the size of the sample was not assessed. All patients with available data were included. Second, owing to the limited sample size, we are not able to conduct subgroup analyses stratified by different medications. Third, we did not replicate the findings in an independent cohort. We will continue the validation work in future research.
Conclusions
The study revealed increased serum CLP in psoriatic patients and its correlation with PsA disease activity. Serum CLP complex possesses potential as a useful biomarker to predict the obtainment of treatment targets in patients with PsA.
References
Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369: m1590.
Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251-65.e19.
Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–90.
Perez-Chada LM, Merola JF. Comorbidities associated with psoriatic arthritis: review and update. Clin Immunol. 2020;214: 108397.
Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–85.
Gialouri CG, Fragoulis GE. Disease activity indices in psoriatic arthritis: current and evolving concepts. Clin Rheumatol. 2021;40(11):4427–35.
Sokolova MV, Simon D, Nas K, Zaiss MM, Luo Y, Zhao Y, et al. A set of serum markers detecting systemic inflammation in psoriatic skin, entheseal, and joint disease in the absence of C-reactive protein and its link to clinical disease manifestations. Arthritis Res Ther. 2020;22(1):26.
Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700–12.
Hagege B, Tan E, Gayraud M, Fautrel B, Gossec L, Mitrovic S. Remission and low disease activity in psoriatic arthritis publications: a systematic literature review with meta-analysis. Rheumatology (Oxford). 2020;59(8):1818–25.
Korndorfer IP, Brueckner F, Skerra A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J Mol Biol. 2007;370(5):887–98.
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665–73.
Schoels MM, Aletaha D, Alasti F, Smolen JS. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis. 2016;75(5):811–8.
Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69(1):48–53.
McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73(2):349–56.
Ometto F, Botsios C, Raffeiner B, Sfriso P, Bernardi L, Todesco S, et al. Methods used to assess remission and low disease activity in rheumatoid arthritis. Autoimmun Rev. 2010;9(3):161–4.
Obry A, Lequerre T, Hardouin J, Boyer O, Fardellone P, Philippe P, et al. Identification of S100A9 as biomarker of responsiveness to the methotrexate/etanercept combination in rheumatoid arthritis using a proteomic approach. PLoS ONE. 2014;9(12): e115800.
Turina MC, Yeremenko N, Paramarta JE, De Rycke L, Baeten D. Calprotectin (S100A8/9) as serum biomarker for clinical response in proof-of-concept trials in axial and peripheral spondyloarthritis. Arthritis Res Ther. 2014;16(4):413.
Benoit S, Toksoy A, Ahlmann M, Schmidt M, Sunderkotter C, Foell D, et al. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br J Dermatol. 2006;155(1):62–6.
Farag AGA, Shoaib MAA, Labeeb AZ, Sleem AS, Hussien H, Elshaib ME, et al. S100A8 (rs3806232) gene polymorphism and S100A8 serum level in psoriasis vulgaris patients: a preliminary study. J Cosmet Dermatol. 2022;2:2.
Aochi S, Tsuji K, Sakaguchi M, Huh N, Tsuda T, Yamanishi K, et al. Markedly elevated serum levels of calcium-binding S100A8/A9 proteins in psoriatic arthritis are due to activated monocytes/macrophages. J Am Acad Dermatol. 2011;64(5):879–87.
Jarlborg M, Courvoisier DS, Lamacchia C, Martinez PL, Mahler M, Bentow C, et al. Serum calprotectin: a promising biomarker in rheumatoid arthritis and axial spondyloarthritis. Arthritis Res Ther. 2020;22(1):105.
Ometto F, Friso L, Astorri D, Botsios C, Raffeiner B, Punzi L, et al. Calprotectin in rheumatic diseases. Exp Biol Med (Maywood). 2017;242(8):859–73.
Wang Q, Chen W, Lin J. The role of calprotectin in rheumatoid arthritis. J Transl Int Med. 2019;7(4):126–31.
Bai S, Wang W, Ye L, Fang L, Dong T, Zhang R, et al. IL-17 stimulates neutrophils to release S100A8/A9 to promote lung epithelial cell apoptosis in Mycoplasma pneumoniae-induced pneumonia in children. Biomed Pharmacother. 2021;143: 112184.
Nakajima K, Kanda T, Takaishi M, Shiga T, Miyoshi K, Nakajima H, et al. Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model. J Immunol. 2011;186(7):4481–9.
Romand X, Bernardy C, Nguyen MVC, Courtier A, Trocme C, Clapasson M, et al. Systemic calprotectin and chronic inflammatory rheumatic diseases. Joint Bone Spine. 2019;86(6):691–8.
Rahman MT, Myles A, Gaur P, Misra R, Aggarwal A. TLR4 endogenous ligand MRP8/14 level in enthesitis-related arthritis and its association with disease activity and TLR4 expression. Rheumatology (Oxford). 2014;53(2):270–4.
Christmann C, Zenker S, Martens L, Hubner J, Loser K, Vogl T, et al. Interleukin 17 promotes expression of alarmins S100A8 and S100A9 during the inflammatory response of keratinocytes. Front Immunol. 2020;11: 599947.
Gialouri CG, Evangelatos G, Pappa M, Karamanakos A, Iliopoulos A, Tektonidou MG, et al. Normal C-reactive protein in active psoriatic arthritis: results from real-world clinical practice. Ther Adv Musculoskelet Dis. 2022;14:175.
Mylemans M, Nevejan L, Van Den Bremt S, Stubbe M, Cruyssen BV, Moulakakis C, et al. Circulating calprotectin as biomarker in neutrophil-related inflammation: pre-analytical recommendations and reference values according to sample type. Clin Chim Acta. 2021;517:149–55.
Acknowledgements
This study was based on the contributions of all colleagues in our department during the outpatient visits over the past decade.
Funding
This work and the journal’s rapid service fee were supported by the interdisciplinary clinical research project of Peking University First Hospital (2021CR30) and the youth clinical research project of Peking University First Hospital (2019CR28).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors contributions
Zhuoli Zhang conceived and designed the study. Borui Li, Zhibo Song, and Guangtao Li collected the data. Borui Li performed the ELISA analysis, performed the statistical analysis, and drafted the manuscript. Zhuoli Zhang also critically revised the manuscript. All the authors read and approved the final manuscript.
Disclosures
Borui Li, Guangtao Li, Zhibo Song, and Zhuoli Zhang have nothing to disclose.
Compliance with Ethics Guidelines
The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments and approved by the ethics committee of Peking University First Hospital. Informed consent was obtained from each patient.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, B., Li, G., Song, Z. et al. Serum Calprotectin as a Promising Inflammatory Biomarker in Psoriatic Arthritis: a 1-Year Longitudinal Study. Rheumatol Ther 10, 149–160 (2023). https://doi.org/10.1007/s40744-022-00501-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-022-00501-5