Abstract
Purpose of Review
Because tree seeds have been considered a low-risk pathway for the spread of plant pathogenic fungi, their international movement is not subject to strict phytosanitary regulation. However, recent studies have provided scientific evidence that the biosecurity risk of seed trade may not be as negligible as assumed. This review summarises current knowledge about seed trade activity across the world and seed-borne plant pathogenic fungi and highlights knowledge gaps that need to be filled to mitigate the risk of spreading tree pathogens via seeds.
Recent Findings
Several outbreaks of severe tree diseases in natural forests and plantations worldwide have been linked to fungal pathogens spread by seed trade. Indeed, recent studies based on modern sequencing technologies have shown that tree seeds harbour highly diverse fungal communities, including well-known pathogens and fungal taxa belonging to unknown species. While it has become clear that even apparently healthy seeds can carry potentially pathogenic fungi, the likelihood of seed-borne pathogens being introduced and becoming established, spreading and causing impact in the new environment is still unclear which challenges the assessment of the phytosanitary risk posed by seed trade.
Summary
Our analyses show that large amounts of tree seeds have been traded among countries and continents. Based on published literature, the risk of spreading pathogenic fungi via tree seed movement is high. However, the role of the taxonomically and functionally diverse fungal communities associated with seeds is still poorly understood. In particular, more research is needed to assess the likelihood of seed-borne fungi being transmitted to the seedlings and spreading and causing impact in the new environment.
Similar content being viewed by others
Introduction
Seeds harbour a highly diverse microbiome consisting of dominant “core taxa” that appear across host species and environments, and of “flexible taxa” which are acquired from the environment to help the offspring adapt to local conditions [1•]. Many of these seed-associated microorganisms are known to be transmitted to the seedlings and to help seedling development, for example by producing antimicrobial compounds and promoting nutrient uptake [2•, 3•]. However, seeds can also harbour potentially pathogenic taxa which may cause diseases when the native host is facing environmental stresses [4] or if introduced to new environments where they may have no natural enemies and/or may encounter naïve hosts [5, 6]. Most of the current knowledge about seed associated microbiota originates from studies on agricultural crops and for verifying its validity for forest tree seeds, more research is needed.
Traditionally, tree seeds have been considered a relatively low risk pathway for the movement of non-native pathogens compared to plants for planting [7]. However, there is increasing evidence that the phytosanitary risks associated with seeds may not be as negligible as assumed since seeds may harbour harmful microorganisms, mainly fungi, threatening tree health [8, 9••]. Seed-borne fungal pathogens can be transported on the surface or within the traded seed [10••, 11••, 12••] as spores or dormant mycelium and include seed-transmitted pathogens that directly infect the host plant growing from the seed and non-seed-transmitted pathogens that are first transferred to the environment (e.g. water, soil) from where they can infect a host plant [13••]. Both groups of seed-borne pathogens can cause poor germination, germination failure or seedling diseases, posing a true concern to nursery owners, foresters and seed traders [12••].
There are only a few known records of fungal pathogens that have been shown to have been introduced into new areas by seeds, and those are mostly pathogens of commercially grown gymnosperms [9•] (Fig. 1). For example, the seed pathogen Geniculodendron pyriforme (syn. Caloscypha fulgens) was imported on spruce (Picea) and fir (Abies) seeds into Germany from the USA and Canada [14]. The introduction of Fusarium circinatum, the causal agent of pine pitch canker, in the southern and northern hemisphere has been linked to the trade of pine (Pinus) seeds [15,16,17]. The seed-borne fungus Neofusicoccum parvum was reported to inhibit the growth of Araucaria seedlings in the southern hemisphere [18] and Sirococcus conigenus is causing mortality on Norway spruce (Picea abies) and noble fir (Abies procera) in Norwegian nurseries [19]. Within the angiosperms, seeds used for the establishment of Eucalyptus plantations have likely served as vectors for the seed-transmitted Eucalyptus stem canker pathogen Teratosphaeria zuluensis [20] and for Quambalaria eucalypti [21] in the southern and northern hemisphere, respectively. Moreover, fungal pathogens, such as those belonging to the genera Lasiodiplodia, Neofusicoccum and Mycosphaerella, were also reported in commercially traded eucalypt seeds [12••]. Recent molecular studies showed that commercially traded tree seeds carry highly diverse fungal communities, including pathogens causing no symptoms [10, 11••, 22•] that may be difficult to detect during border inspections, demonstrating the hidden biosecurity risks of international tree seed movement.
Diversity of seed-borne fungi. A Seed-borne fungi growing from Cedrus atlantica seeds on an artificial medium (i.e. potato dextrose agar (PDA) amended with streptomycin sulphate). B Morphotyping (grouping based on the morphology) of fungal isolates growing on PDA plates. C–F Isolates of common seed-borne pathogens of gymnosperms on PDA plates. C Diplodia sapinea, the causal agent of Diplodia tip blight. D Fusarium circinatum, the pine pitch canker fungus. E Neonectria neomacrospora, a causal agent of Neonectria canker of fir. F Sydowia polyspora, a pathogen of several gymnosperm species. Photo credit: Ana Perez Sierra
Currently, around 30% of the European forests are artificially regenerated [23], mainly using seeds from domestic sources or from other European Union (EU) countries [24•]. On average, around 400,000 kg of seeds are traded annually within Europe [24•], although the real amount is probably higher due to un-reported non-EU imports [24•]. Most seeds for forestry are exchanged through certified traders, and national and international guidelines and policies have been adopted to safeguard the trade of forest regeneration material (FRM) [25, 26]. However, while cross-border transfers of FRM must be reported, records are kept at national levels and an EU-wide harmonised documentation has not been established yet; trade flows of FRM are not recorded in any official international database and must be acquired from respective national authorities, which themselves do not always keep an ideal track of the data [24•]. Therefore, the lack of unified international tracking system makes it difficult to estimate the volumes and track the flows of international tree seed trade. Furthermore, existing legislation mostly focuses on regulating the quality and environmental suitability of seed sources rather than the potential harmful organisms that seeds might carry. Seeds are also increasingly bought via E-commerce, over the internet, especially by private users, but E-commerce is rarely factored into risk analysis by National Plant Protection Organisations (NPPOs). Plants (including seeds) traded in E-commerce are often shipped in small quantities and do not belong to easily recognisable consigments (e.g. small packages of seeds, seed-infused greeting cards, bookmarks), while phytosanitary frameworks were designed to target trade of bulk and easily identifiable commodities [27]. E-commerce has raised growing concerns in the phytosanitary community, and recommendations to NPPOs have been issued by the Commision on Phytosanitary Measures [28] to tackle this new phenomenon. Limited traceability and phytosanitary regulation of seed trade raise concern about the lack of knowledge about seed-borne pathogens that could unintentionally be introduced into new areas and have an impact on nursery stocks and on new or existent forests.
The overall aim of this article is to describe the current knowledge about seed-borne fungi and seed trade as a pathway for fungal pathogens of woody plants. Based on published literature and original research on international tree seed movement, the likelihood of seed-borne fungi to be transported and introduced to a new area, to become established, spread and cause impact is discussed in detail following a proposed scheme (Fig. 2), as a successful completion of each of these steps is necessary for a pest/pathway being considered as high risk and for triggering the introduction of phytosanitary measures to restrict the trade. Existing and possible management options for risk mitigation are also discussed. Finally, the review highlights the existing knowledge gaps that need to be addressed to understand the phytosanitary risks posed by the movement of potentially contaminated seeds.
Invasion framework for seed-borne fungal pathogens. In the exporting country, seeds (and associated pathogens) are collected in seed orchards and seed stands, cleaned, dried and stored. After transport to the importing country, seed-borne pathogens may be introduced into nurseries or, probably less frequently, forests where they may establish and further spread. Infected seedlings may also be transported from a nursery to a forest. Created with BioRender.com (https://biorender.com/)
Transport of Pathogens into a New Country
Although seeds and associated pathogens can be spread over long distances by natural means including large and/or migratory animals, especially birds, extreme meteorological events and even ocean currents ([29] and references within), their main means of spread is human activity, in particular seed trade. Prior to shipment, seeds are collected in selected orchards or forest stands, processed (e.g. cleaning, drying, storing) and eventually stored in seed production facilities (Fig. 2). Thereafter, they can then be either used within national borders or shipped to another country.
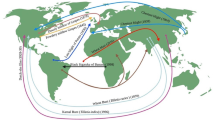
Based on the data on the annual trade in tree seeds between 38 countries collected from Trade Map© [30] by co-authors of this manuscript, approximately 40 million kg of tree seeds for sowing were traded during the period 2005 to 2019 (i.e. 2.7 million kg per year on average), with varying amounts being exchanged within and between biogeographic regions and continents across years (Fig. 3). Before 2010, more than 85% of the seed exchange occurred within Europe and mainly between countries within the same biogeographic region (i.e. 9 million kg within south Europe and 1.2 million kg within north, west and central Europe; Fig. 3a). After 2010, intercontinental trade intensified and reached almost 50% of total exchanged seeds during the years 2010–2014 with large amounts of seeds being exported from North America to south Europe (i.e. 2.3 million kg), north, west and central Europe (i.e. 390 thousand kg) and to East Asia (i.e. 480 thousand kg), and from east and west Asia to north, west and central Europe (i.e. 550 thousand kg; Fig. 3b). Within Europe, large amounts of seeds were exchanged within north, west and central Europe (i.e. 2.7 million kg) and between north, west and central Europe and south Europe (i.e. 1.1 million kg; Fig. 3b). Most recently, between 2015 and 2019 trade within Europe decreased to less than 2 million kg (i.e. 12% of the total traded seeds; Fig. 3c). Most of the seed trade was intercontinental; around 4.5 million kg was exported from North America to south Europe and around 7.7 million kg from south Europe to west Asia. In addition, 400 thousand kg was imported into north, west and central Europe, and a similar amount into west Asia, from North America and around 500 thousand kg of seeds were exported from East Asia to northwest and central Europe. Seed trade between biogeographic regions and continents is thus higher than previously reported [24] and still increasing. This trend is of particular phytosanitary concern because seed-borne fungi were previously shown to be continent specific [10••]. Hence, intercontinental movement might introduce new non-native pathogens with the greatest risk of impact [32], especially when large amounts of seeds are imported from new trading partners from highly diverse regions [33]. This was for example the case of Diplodia pinea, a seed-borne pathogen that was most likely introduced in pine plantations in the Southern hemisphere via seeds. A study by Burgess and Wingfield [16] showed that the genetic diversity of D. pinea in South Africa is higher than in Australia. Noteworthy, in South Africa pine seeds of various origins were imported over a long period, whereas in Australia seeds were mostly locally sourced. The genotypic diversity of the pathogen in New Zealand was moderate reflecting the free imports before 1930s and stricter import regulations afterwards. Besides linking the introduction of this pathogen to seed imports, the authors emphasise the importance of phytosanitary measures to mitigate the damage posed by non-native pathogens.
Amounts of tree seeds for sowing traded between continents, and between biogeographic regions in Europe, during three time periods: 2005–2009 (a), 2010–2014 (b) and 2015–2019 (c). Sums of exchanged seeds during each time period are shown in brackets. Sankey plots were generated using sankeyNetwork function from sankeyD3 package [31] in R. Tree seed trade data was extracted on annual weight in kilograms for 25 countries for years 2005 through to 2019 from Trade Map© [30] based on Harmonised System codes used in the trade of forest tree seed for sowing: “12099910 forest tree seeds for sowing (Austria, Belgium, Croatia, Czech Republic, Denmark, Finland, France, Germany, Hungary, Italy, Luxembourg, Netherlands, Poland, Slovenia, Spain, Sweden, Georgia, Türkiye, UK)”, “12099910 tree and flower seeds, of a kind used for sowing” (Australia), “1209991000 seeds, fruit and spores for sowing, nes: forest tree seeds (Serbia)”, “1209990051 seed; tree, of a kind used for sowing” (New Zealand), “1209991010 Caucasian fir seeds (Abies nordmanniana (stev.) spach)” (Russian Federation), “1209991010 tree seeds, for sowing, other than nut trees of chapter 8” (Canada), “1209992000 tree and shrub seeds, fruits and spores, of a kind used” (USA). Data for China, Greece, Hong Kong, Ireland, Israel, Japan, Mexico, Norway, Portugal, Republic of Korea, Romania, Slovakia and Switzerland were derived from the extracted data—they could not be extracted directly because of the insufficiently precise codes. When the reported export and import volumes for a combination of countries did not equate, the maximum recorded volume was taken as the value. Colours correspond to continents: North America (Canada, Mexico, USA), blue; West Asia (Georgia, Israel, Türkiye), red; East Asia (China, Hong Kong, Japan, Republic of Korea), orange; Oceania (Australia and New Zealand), yellow, and biogeographic regions within Europe are shown in the shades of green as North, West and Central Europe (Austria, Belgium, Czechia, Denmark, France, Germany, Ireland, Luxembourg, Netherlands, Switzerland, UK), Northeast Europe (Finland, Norway, Poland, Russian Federation, Sweden), Southeast Europe (Croatia, Hungary, Romania, Serbia, Slovenia, Slovakia), South Europe (Spain, Greece, Italy, Portugal)
Besides seed origin and seed volume, the risk of transporting pathogenic fungi with traded tree seeds is affected by tree species. In the recent study by Franić et al. [10••], 58 traded seed lots of 11 gymnosperm and angiosperm tree species from North America, Europe and Asia were assessed for the presence of fungi using traditional culturing and high-throughput sequencing (HTS). Although the investigated seeds appeared healthy, fungi were cultured from 96% of the seed lots. The incidence within lots varied considerably across species (e.g. 5% of infected seeds in P. abies versus 96% of infected seeds in Fagus sylvatica). Strong inter- and intra-specific variation in the incidence of potential pathogens cultured from seeds was also reported in other studies (e.g. examples for Diplodia sapinea in Decourcelle et al. [34] and references within, and several fungi, including Sydowia polyspora isolated from 12 seed lots of three Abies species from five different countries in Talgø et al. [35]). In Franić et al. [10••], the HTS approach revealed potential pathogens from all examined seed lots. Potentially, pathogenic fungi comprised around 50% and 30% of the total fungal community in the seeds of angiosperms and gymnosperms, respectively, indicating that the trade of angiosperm seeds might represent a higher phytosanitary risk than gymnosperm trade. However, even when the incidence of seed-borne pathogens within the seed lot is low, the risk of their transport may not be negligible as seeds are usually traded in large amounts.
The mode of the long-distance seed transport (e.g. sea, air, road, rail) probably does not affect chances of survival and successful arrival of seed-borne pathogens. Between continents, the transport is most likely to occur mainly by sea. For example, in 2021, sea transport accounted for 48% of valued goods traded between the EU and the rest of the world, measured in volume the share was 74% [36]. Within continents, transport might rely on other modes of transport. In any case, seeds are transported in the way to ensure their viability post transport, which includes relatively cold and dry conditions, similar as those applied for seed storage (see below). Nowadays, intercontinental trips are relatively short—it takes 10–20 days for a cargo ship to cross the Atlantic which insures minimum impact on seed viability and on the survival of seed-borne fungi.
Introduction of Pathogens into a New Environment
After arrival to a new country, seeds might be kept in storage until being sown in the nursery or, eventually, in the forest stand (Fig. 2). Most tree seeds can be stored for several years at low moisture contents. For example, orthodox seeds such as those of Pinus and Picea species and most other temperate woody species can be stored at 6–8% moisture content at 3–5 °C, while intermediate seeds such as those of Abies, Cedrus and most broadleaved species can be stored at 10–15% moisture content at temperatures between −10 and 20 °C [37]. Recalcitrant seeds, for example of Quercus, Castanea and Acer, begin to die below 40% moisture content and should be stored at 3–5 °C [37]. However, even at the right conditions, recalcitrant seeds rapidly lose their viability and should ideally be sown immediately upon collection. Relatively dry and cold conditions used for the storage of tree seeds might have only little effect on seed-borne pathogens, as several pathogenic fungi can survive long storage periods. For example, the common storage pathogen C. fulgens, which infects and kills seeds of Picea, Abies and Pinus species, as well as of Douglas fir (Pseudotsuga menziesii), can grow at temperatures from −1 to 27 °C and spread from diseased to healthy seeds under cold and moist conditions [38]. Moreover, C. fulgens can survive long storage periods, as shown by Schröder et al. [14], who isolated the fungus from Abies and Picea seeds after 3 and 4 years of storage, respectively. Other seed-borne fungi which do not kill the seeds, but might cause diseases of seedlings, can survive long periods under storage conditions. For example, seven seed-borne Fusarium species survived storage of Pinus ponderosa seeds for one to 2 years at 10–11% moisture content and 2–3 °C [39]. The pine pitch canker pathogen F. circinatum was isolated after five months from Pinus palustris seeds stored at 6–7% moisture level and 5 °C [40]. Moreover, a low incidence of D. sapinea (i.e. 0.7–9%) was demonstrated in commercially extracted Pinus nigra seeds stored at 3–4 °C and 7–10% moisture level for 1 to 3 years [34]. The pathogen S. conigenus survived in naturally infected P. abies seeds at 5–8% moisture content during 3 years’ storage at 2 °C [19], although its incidence was very low [19]. In summary, seed storage under low moisture content and cool temperatures may keep the infection levels low, but it does not kill seed-borne fungi. Noteworthy, commercial seed extraction from cones includes the application of dry heat for cone opening, which might explain the low incidence of pathogens in storage [34]. However, in production nurseries even low levels of inoculum can be considered significant because of the high density of the seedlings and frequent irrigation which results in optimal humidity for fungal development [19].
Fungal pathogens which survive an initial storage period before sowing, will finally be introduced to the new environment when seeds are sown in the nursery or, eventually, directly in the forest stand (Fig. 2). The majority of seed-borne pathogens seem to be first transferred from seeds to the abiotic environment (e.g. water, soil) from where they can infect a host plant (i.e. non-seed-transmitted pathogens). Although a high fraction of seed-associated fungi seems to be transferred to seedlings [2•, 3•], only a few tree seed-borne pathogens have been proven to be seed-transmitted [9••]. For example, transmission from seeds to seedlings was reported for S. conigenus in spruce [41], F. circinatum in Douglas fir [42] and pine [43, 44] and F. oxysporum in Douglas fir [42]. In other cases, like for D. sapinea, the demonstration of transmission of fungal pathogens by seeds remains unclear [16, 45]. Since seed-transmitted fungi have higher chances of establishment in the new environment than non-seed-transmitted fungi, more studies are needed to determine the prevalence of seed transmission among tree seed-borne fungi.
Establishment of Pathogens in the New Environment
After its introduction and during the establishment phase, a seed-borne pathogen must be able to survive in the new environment. Some pathogens are likely to remain in/on the seeds where they can produce spores capable of infecting living plants. For example, conidia of the seed-borne fungus Marssonina brunnea, a causal agent of poplar anthracnose, can survive on the surface of poplar seeds from where it can initiate seedling infections [46]. Other pathogens can colonise woody debris present in the soil or can be released into the soil and survive as resting structures awaiting appropriate abiotic conditions for infection.
The likelihood of survival of seed-transmitted pathogens in the plant will depend on how they interact with the endophyte community present both in seeds, and later in seedlings [47]. Some endophytes may exert pressure on the pathogen helping the host plant to better resist infection [48]. Others can help the host plant defence by acting directly on the plant’s metabolism [48], or by placing the plant into a better developmental conditions (mycorrhiza in particular). Similarly, pathogens surviving in soil debris will have to compete with other soilborne saprobionts that might be more successful in utilising nutrients and colonizing the soil [49], or could be suppressed by soil microorganisms producing fungistatic compounds [50].
If a seed-borne fungal pathogen survives in the new environment, to become established locally, it must be able to infect new hosts or substrates. Since spores are the main infection propagules of fungi, infection capacity of a pathogen depends mostly on its sporulation ability, which is a complex trait influenced by several abiotic factors, including temperature, moisture, availability of food and light conditions. Temperature affects the species-specific number of spores formed and released, and humidity plays an important role in spore formation, release, longevity and spread, and is essential for spore germination. For example, in F. circinatum, a higher air inoculum is favoured by lower air temperatures during the days before spore release [51] and spore dispersal is adapted to splashing water [52]. High winter temperatures and abundant rain during late summer promote spore production in D. sapinea [53], whereas spores of S. conigenus can survive temperatures of −10 °C, but not above 35 °C [54]. Light/radiation is also known to have a direct influence on spore production, survival and germination. Fungal species able to rapidly produce high amounts of asexual spores theoretically have the best chances to infect new hosts.
The ability of a fungal pathogen to infect new plants depends on its degree of host specialisation. The broader the host range of a pathogen, the higher the probability it may encounter a suited host plant. While tree seeds are known to carry ubiquitous potential pathogens such as Epiccocum nigrum or Alternaria spp., they are in general highly host specific [22•, 55•]. In addition to the infection of plants of the known hosts, infections of new host species may occur in the new environment. This host shift will depend on several factors including pre-adaptation of the pathogen, evolutionary change, likelihood of contact between the current and the new host or the presence of other pathogens facilitating the new interaction [56, 57]. Reports of F. circinatum damage in an increasing number of Pinus species after its introduction in different regions of the world, as well as its presence in grass and herb species [17], may indicate a potential host shift for this pathogen.
Host-related factors such as availability, density and susceptibility in the new environment are also crucial for the successful establishment of seed-borne pathogens [22•, 56, 58, 59]. Potential host plants should be available in proximity of the primary inoculum, which will often remain in the plant, in the soil or on plant debris near the site of introduction. Host density in the surroundings may influence spatial dynamics of pathogen’s establishment [60]. Finally, host susceptibility depends on the history of host-pathogen interaction. Hosts lacking specific resistance genes will be more prone to infection and damage development than co-evolved hosts. However, if both the host and its co-evolved pathogen are introduced into a new location (i.e. “pathogen reunion”) [61], either together or separately, the new environmental conditions might be conductive to disease development while this was not the case in the native region [62].
Once a fungal pathogen has infected a suitable host in the new environment, it must be able to persist locally and constitute a viable population to avoid extinction [61, 63]. At this stage of the invasion process, the population of the pathogen might be still limited in size and genetic diversity. The low population density may be associated with Allee effects, i.e. a reduced population growth and fitness that could decrease chances of survival for the population. However, multiple introductions from genetically different source populations, or sexual reproduction increasing genetic diversity may counteract the Allee effects.
Spread of Pathogens in a New Environment
After establishment, to spread in the new environment and start a new independent population (Fig. 2), seed-borne fungi must be able to exit the primary population, which may occur with or without their active involvement. Passive dispersal may be due to animals or humans which accidentally (e.g. mechanical contact) or on purpose (e.g. feeding) spread parts of the seedlings over long distances. Active spread usually involves the production of spores, which then act as dispersal propagules. Sporulation of endophytic fungi present in the seedlings may be stimulated by biotic (e.g. pests or pathogens, competition with other seedlings) and abiotic (e.g. drought, light deficiency) stresses which weaken the seedling and give an advantage to the associated fungi. For instance, outbreaks of D. sapinea are generally associated with stress factors such as drought, hail damage or excess nitrogen [64]. Milder winters over the last 25 years have most likely allowed the pathogen to expand its range towards northern Europe, threatening an even larger number of conifer species [65, 66].
Most fungal spores are dispersed by wind, but spores may also be spread by water or animals (e.g. insects, birds). For example, twig-mining beetles such as Phloeosinus aubei, P. thujae and P. armatus, which are common in the Mediterranean area, may have played a significant role in the spread of the seed-borne cypress canker pathogen Seiridium cardinale [67]. If the infected trees in the primary population are already fructifying, spread of seed-borne fungi may also occur via insects feeding on cones and seeds (e.g. association between the seed bug Orsillus maculatus and the seed chalcid Megastigmus wachtli, and the cypress canker pathogen [68], or between the western conifer seed bug Leptoglossus occidentalis and D. pinea on Pinus pinea [69]). Hoover et al. [70] showed that Conophthorus radiatae and Ernohius punctulatus, both infesting cones and twigs of P. radiata in California, can transmit F. circinatum to healthy cones under experimental conditions. Besides acting as vectors, insects, in particular bark beetles, may also wound the trees with their breeding and feeding galleries, thereby promoting fungal infection [71]. Finally, humans or animals may also spread seed-borne fungi by moving around infected seeds produced in the primary population. Although the chestnut blight fungus Cryphonectria parasitica is not well known as a seed-borne pathogen, Jaynes and DePalma [72] found out that about 14% of the nuts harvested from a planting of American chestnuts (Castanea dentata) in Connecticut (USA) were actually infected with the pathogen. In Portugal, F. circinatum was first detected in 2007 in a nursery [73]. In 2016, a seed lot originating from a Pinus radiata stand in the north of the country was tested positive.
Typically, fungi dispersed by rain splash are spread over short distances, while wind-dispersed fungi have the largest dispersal potential. High concentrations of airborne spores of F. circinatum have been detected up to 1000 m following the wind direction from infected P. radiata trees [51], even if such spores are borne in a viscous liquid and seem to be better suited to dispersal by water or attached to motile vectors than to aerial dispersal [74]. New populations may become established far from the source population and, with time, will eventually merge with it to significantly advance the population front [58].
Following spore dispersal, several conditions must be right for a new fungal population to be founded. First, climate conditions must be suited for spore survival, germination and development of a persistent fungal colony. Although in Chile F. circinatum is present in nurseries, it has never spread and established in plantations, most likely because of suboptimal climatic conditions [75]. For germination, spores need to find suitable host(s), which may be of the same species as the one(s) in the primary population or eventually belong to different species (in the extreme case, a host jump may occur). Similar as for establishment, specific factors of the host population may further affect chances of successfully developing a persistent secondary fungal population [58]. For example, Skulason et al. [76] found that the damage caused on subalpine fir (Abies lasiocarpa) by Neonectria neomacrospora, a canker-causing fungus also found on seeds, varied depending on the origin of the trees. In a survey conducted in central Italy, Danti et al. [77] observed that incidence and severity of S. cardinale on Leyland cypress were higher in older plantations and explained it with the increased presence of bark cracks in older trunks favouring the entrance of the pathogen. At the landscape scale, spread dynamic of fungi is strongly influenced by variations in landscape structure, such as topography, geographic/environmental barriers and forest fragmentation [58], as well as by the presence/absence of potential competitors, hyperparasites or predators. If a seed-borne pathogen succeeds in spreading and establishing a new population, its impact may be particularly severe. For example, after its introduction S. cardinale has caused severe epidemics in forests, plantations and ornamental cypress trees in several countries around the world [78].
Phytosanitary Management of Tree Seeds
A substantial proportion of the international trade in forest tree seeds occurs within biogeographical regions such as Europe or North America, but seeds are also increasingly traded between biogeographical regions (Fig. 3). It is this trade between biogeographical regions that presents the greatest risks of invasive species impacts [32]. At international level, the Organisation for Economic Cooperation and Development (OECD) Forest Seed and Plant Scheme facilitates the international trade of forest reproductive material through harmonised standards certifying its provenance, identity, quality and traceability, thus contributing to the sustainable use of valuable forest genetic resources [26]. The market of forest reproductive material in the EU is regulated by national laws, based on the Council Directive 1999/105 [25]. However, these regulations do not aim at mitigating the phytosanitary risk of seed trade. Phytosanitary regulations include Regulation 2016/2031 [79] and Commission Implementing Regulation (EU) 2019/2072 [80] based on the International Plant Protection Convention (IPPC) which reports the list of plants, plant products and other objects whose introduction into the EU from certain third countries is prohibited and other measures that should be taken to reduce the risk of plant trade. However, tree seeds, except pine (Pinus) and Douglas fir (Pseudotsuga menziesii) seeds, are exempted from most plant trade restrictions. Nevertheless, an increasing number of EU countries and regions are adopting and implementing measures that may help to reduce, or in some cases mitigate, the phytosanitary risk associated to seed trade.
Risk Reduction Measures
Measures that reduce rather than mitigate risk aim at reducing the likelihood of a pathogen establishing in a new area. Under the IPPC, contracting parties may require traded forest tree seeds to be accompanied by a phytosanitary certificate [81]. Until recently, most of the seed imports to Europe were exempted from this requirement, but recently phytosanitary certificates have become obligatory for all seeds imported to Europe [80]. The phytosanitary certificate includes a declaration that “the plants, plant products or other regulated articles described herein have been inspected and/or tested according to appropriate official procedures and are considered to be free from the quarantine pests specified by the importing contracting party […]” and “They are deemed to be practically free from other pests” [81]. While the terms “free from” or “practically free from” would indicate risk mitigation, the use of inspection and/or testing to confirm freedom limits the effectiveness of this measure.
Inspecting a consignment of seeds for pathogens has limited efficacy mainly due to the lack of symptoms of an infection [82, 83]. Many pathogens have no visible impact on seed phenology if the seed reaches maturity (e.g. pathogen infection occurs late in seed development). For example, F. circinatum, the only quarantine pathogen in Europe for which seed is recognised as a pathway does not cause any symptoms in pine and Douglas fir seeds and cannot be detected by visual examination [84]. Tree seeds are traded in large consignments and inspections, for practical reasons, only target subsamples. Depending on the method used for pathogen identification, the number of seeds to be tested per seed lot to detect a pathogen at different levels of infection in the lot may vary and should be determined statistically [85]. If the pathogens of concern are known, species-specific tests can be conducted. In the case of F. circinatum, detailed instructions on plating and molecular approaches are given in the International Standard for Phytosanitary Measures (ISPM) 27 [74] and the EPPO standard describing a diagnostic protocol for F. circinatum [84]. The recommended sample size by the International Seed Testing Association (ISTA) is at least 400 seeds [86]. However, larger samples (e.g. 1000 seeds) can be processed for detection of F. circinatum by biological enrichment prior to DNA analysis using quantitative polymerase chain reaction (qPCR) [87].
Recently, new sequencing technologies (e.g. HTS) have been applied to assess the seed-borne mycobiome, which have shown to be more powerful than traditional plating [10, 11]. HTS methods could be used for robust, reliable and cost-effective detection of regulated pests as well as of unknown organisms (i.e. without a priori knowledge) in the inspection of plant material for certification, quarantine testing or monitoring of imported commodities for new potential pests [88]. Targeting RNA instead of DNA molecules or sequencing longer DNA fragments would ensure that only viable organisms are revealed from biological samples [89]. However, the implementation of such technologies still faces some challenges (e.g. requirements for laboratory infrastructure, bioinformatics, data sharing and validation, standardised protocols) [88]. For example, sensitivity and specificity levels associated with these approaches may not yet be known; Oskay et al. [90•] found that an increase in number of seeds used for DNA extraction resulted in more fungal taxa revealed from a sample. Thus, more research is needed to establish standardised HTS protocols for phytosanitary purposes. In this regard, EPPO recently published a standard on the use of HTS in plant health diagnostics based on the recommendations given by ICCP [88]. This, in order to develop best-practice operational guidelines and to ensure robust and accurate data output, biological significance of the results in a phytosanitary context, harmonized implementation, test validation and quality assurance [88].
Risk Mitigation Measures
Measures that aim to mitigate the risk of pathogen introduction from traded forest tree seed take two forms: preventing seed exposure before harvest or treating the traded seeds to kill potential pathogens. The most stringent measure, prohibition of import if the risk is considered inacceptable and no effective mitigation measures are known, has not yet been applied to forest tree seeds. The measure for preventing seed exposure before harvest is “area freedom”, i.e. seeds have to be harvested from production areas free of a pathogen [91]. This specific measure effectively mitigates phytosanitary risk when the pathogen of concern is known. For example, in 2007, the Commission Decision 2007/433/EC (EC433 2007) specified that plants of the genus Pinus and the species P. menziesii intended for planting, including seeds and cones for propagation purposes, might be moved within the EU only if they are accompanied by a plant passport prepared and issued in accordance with the provisions of Commission Directive 92/105/EEC (EEC105 1992) and they have been grown throughout their life or since their introduction into the EU in a) a place of production of a Member State where the organism is not known to occur, or b) a pest-free area, established by the responsible official authority in a Member State, or c) where no signs of the pathogen have been observed during official surveys carried out within 2 years before the movement and have been tested immediately before the transfer. As previously mentioned, while the risk of seeds carrying F. circinatum has been recognised and seeds of pines and Douglas fir have consequently been regulated for trade, many unknown and unregulated potential pathogens can be moved to new areas with traded seeds [10••, 11••].
The second form of risk mitigation measures involves the use of treatments to kill the pathogens. Currently available treatment options include the application of chemicals as liquids, powders or as a fumigant. Chemical treatment of seeds can be used to eliminate surface contaminations by pathogens and in some cases prevent or remove infection of any resulting seedling. Common treatments against pathogens living on the seed surface include immersion in a 1% sodium hypochlorite solution for 10 min [92, 93], whereas treatments against internally borne pathogens include the application of slurries of Captan (active ingredient is penconazole (triazole)) or Thiram (active ingredient is thiram (thiocarbamate)) [94]. While chemicals may be effective against some specific pathogens, they cannot target all fungal species found in association with tree seeds [95]. Moreover, chemical methods are not always environmentally friendly. For this reason, they have been increasingly replaced by non-chemical methods, such as treatment with biopesticides and application of irradiation or heat [96].
The use of non-chemical treatments is limited by their effectiveness and their impact on seed viability. For example, irradiation is effective at killing fungal pathogens at doses of 2–5 kGy [97], but such rates will significantly reduce seed viability [98, 99], thereby limiting the practical use of irradiation as a treatment option. Plant pathogens are more susceptible to heat requiring treatments of 60–70 °C for around 2 h [100]. While tree seed tolerance to heat varies, seed from genera such as Pinus are relatively heat tolerant [101]. The use of hot water treatment at 51–52 °C for 30 min was able to reduce F. circinatum contamination on P. radiata seeds [102]. A combination of chemical and heat treatments was tested by Berbegal et al. [103] to reduce inoculum levels of F. circinatum on pine seeds. The study revealed that hot water-treated seeds was a better alternative to hydrogen peroxide and fungicides and could easily be implemented as standard practice in commercial nurseries to minimise the spread of the pathogen. Australia provides heat treatment options for internally borne pathogens of coniferous tree seeds at 54 °C for 86 h, 60 °C for 24 h or 66 °C for 8 h [93]. Similarly, it has been shown that heat treatment at 55 °C for 8, 9, 10 and 11 h can completely eliminate pathogenic fungi such as D. sapinea and F. circinatum from P. radiata seed coat, embryo and gametophyte, without adversely affecting seed germination [104]. Heat treatment could thus be a promising technique to be applied during seed production or pre/post transport to reduce the infestation rate of traded seed lots.
Risk mitigation measures, including chemical and non-chemical seed treatments, aim to produce sterile (i.e. pathogen-free) seeds which have traditionally been considered as healthy. However, such practices may also destroy the beneficial fraction of the seed microbiome that is transferred to the progeny [3•]. Hence, instead of aiming at sterilising the seeds, future solutions might include the manipulation of seed microbiome for enhancing (a)biotic stress resistance, such as a combination of breeding of new cultivars with optimal microbial communities and an active introduction of beneficial microbes to the seeds, as previously suggested for crop cultivation [105].
Conclusions
Plant health regulations and phytosanitary measures aim to reduce the risks of spreading harmful organisms that could represent a threat for agriculture, horticulture and forestry. In this context, seeds have been considered one of the safest ways to introduce plant species into new areas and consequently their trade is regulated less strictly. The high demand of seeds for forest regeneration purposes results in huge volumes of seeds being moved across countries and continents, with variable patterns over time. As shown by recent studies, tree seeds can harbour taxonomically and functionally diverse fungal communities, including potentially pathogenic species. Hence, the global seed trade could theoretically represent a high risk of introduction of potential plant pathogens into new areas. Nevertheless, comprehensive investigations about seeds as vector for fungal pathogens are still missing as seeds have been proven to be a pathway for only five tree pathogens. In addition, detailed studies are needed to assess if and how frequently seed-borne fungi are transmitted to developing seedlings and if from there they can establish in the new environment and cause damage to the local tree communities.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Simonin M, Briand M, Chesneau G, Rochefort A, Marais C, Sarniguet A, et al. Seed microbiota revealed by a large-scale meta-analysis including 50 plant species. New Phytol. 2022;234:1448–63. Paper characterizes core and flexible seed microbiota using 63 studies covering 50 plant species to help uncover roles of seed microbiota for plant health and to design effective microbiome engineering.
• Abdelfattah A, Wisniewski M, Schena L, AJM T. Experimental evidence of microbial inheritance in plants and transmission routes from seed to phyllosphere and root. Environ Microbiol. 2021;23:2199–214. Paper demonstrates high diversity and spatial separation of the fungal and bacterial communities within seeds and seedlings of the pedunculate oak Quercus robur. The results indicate inheritance, niche differentiation and divergent transmission routes for the establishment of root and phyllosphere communities.
• Abdelfattah A, Tack AJM, Lobato C, Wassermann B, Berg G. From seed to seed: the role of microbial inheritance in the assembly of the plant microbiome. Trends Microbiol. 2023;31:346–55. Paper discusses the factors affecting the assembly of the microbiome during the microbial inheritance process, highlights future research directions and emphasizes the scientific and societal implications of microbial inheritance.
Slippers B, Wingfield MJ. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Rev. 2007;21:90–106.
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ. Is invasion success explained by the enemy release hypothesis? Ecol Lett. 2004;7:721–33.
Müller MM, Hamberg L, Hantula J. The susceptibility of European tree species to invasive Asian pathogens: a literature based analysis. Biol Invasions. 2016;18:2841–51.
Santini A, Ghelardini L, De Pace C, Desprez-Loustau ML, Capretti P, Chandelier A, et al. Biogeographical patterns and determinants of invasion by forest pathogens in Europe. New Phytol. 2013;197:238–50.
Mittal RK, Anderson RL, Mathur SB. Microorganisms associated with tree seeds: world checklist 1990. Chalk River (Ontario) Canada: Information Report PI-X-96.; 1990.
•• Denancé N, Grimault V. Seed pathway for pest dissemination: the ISTA Reference Pest List, a bibliographic resource in non-vegetable crops. EPPO Bull. 2022;52:434–45. Paper presents recently established International Seed Testing Association (ISTA) Reference Pest List—an evolving, free online tool, which contains information on numerous seed-borne or seed-contaminating pests of non-vegetable seeds.
•• Franić I, Prospero S, Hartmann M, Allan E, Auger-Rozenberg M-A, Grünwald NJ, et al. Are traded forest tree seeds a potential source of nonnative pests? Ecol Appl. 2019;29:e01971. Paper shows that seeds of 11 angiosperm and gymnosperm tree species obtained from commercial seed suppliers from three continents (i.e. Europe, North America and Asia) serve as a pathway for the introduction of non-native insect pests and fungal pathogens
•• Cleary M, Oskay F, Doğmuş HT, Woodward S, Vettraino AM. Cryptic risks to forest biosecurity associated with the global movement of commercial seed. Forests. 2019;10:459. Paper shows that Pinaceae seeds can serve as a pathway for introduction of native and non-native fungal pathogens.
•• Mangwende E, Chirwa PW, Aveling TAS. Seed health status and germination of Eucalyptus spp. Eur J Plant Pathol. 2021;159:55–65. Paper shows that Eucalyptus seeds can serve as a pathway for the introduction of plant pathogenic fungi which can impact seedling health.
•• FAO. ISPM 38. International movement of seeds. Rome: IPPC, FAO; 2021. This standard provides guidance for NPPOs in identifying, assessing and managing the pest risk associated with the international movement of seeds. The standard also provides guidance on procedures to establish phytosanitary import requirements, on inspection, sampling and testing of seeds and on the phytosanitary certification of seeds for export.
Schröder T, Kehr R, Hüttermann A. First report of the seed-pathogen Geniculodendron pyriforme, the imperfect state of the ascomycete Caloscypha fulgens, on imported conifer seeds in Germany. For Pathol. 2002;32:225–30.
Berbegal M, Armengol J, Grünwald NJ. Evidence for multiple introductions and clonality in Spanish populations of Fusarium circinatum. Phytopathology. 2013;103:851–61.
Burgess T, Wingfield MJ. Quarantine is important in restricting the spread of exotic seed-borne tree pathogens in the southern hemisphere. Int For Rev. 2002;4:56–65.
Drenkhan R, Ganley B, Martín-García J, Vahalík P, Adamson K, Adamcíková K, et al. Global geographic distribution and host range of Fusarium circinatum, the causal agent of pine pitch canker. Forests. 2020;11:1–40.
Dalmas FR, Astarita L, Defilippis L, Magel E, Fiedler H, Bauer R, et al. Growth inhibition of an Araucaria angustifolia (Coniferopsida) fungal seed pathogen, Neofusicoccum parvum, by soil streptomycetes. BMC Microbiol. 2013;13:168.
Brodal G, Bye HR, Høst E, Pettersson M, Fløistad IS, Edvardsen ØM, et al. Management of seed-borne Sirococcus conigenus on Norway spruce by fungicide seed treatment. Seed Sci Technol. 2020;48:33–9.
Jimu L, Kemler M, Wingfield MJ, Mwenje E, Roux J. The Eucalyptus stem canker pathogen Teratosphaeria zuluensis detected in seed samples. Forestry. 2016;89:316–24.
Bragança H, Diogo ELF, Neves L, Valente C, Araújo C, Bonifácio L, et al. Quambalaria eucalypti a pathogen of Eucalyptus globulus newly reported in Portugal and in Europe. For Pathol. 2016;46:67–75.
• Franić I, Eschen R, Allan E, Hartmann M, Schneider S, Prospero S. Drivers of richness and community composition of fungal endophytes of tree seeds. FEMS Microbiol Ecol. 2020;96:1–10. The study shows that host identity is more important than the environment in shaping fungal communities of seeds of several angiosperm and gymnosperm tree species suggesting that although seed trade may facilitate movements of fungi, their establishment and spread in the new environment might be limited by host availability.
FOREST EUROPE. State of Europe’s Forests. Spain: FOREST EUROPE Liaison Unit; 2015.
• Jansen S, Konrad H, Geburek T. Crossing borders – European forest reproductive material moving in trade. J Environ Manage. 2019;233:308–20.
Council Directive 1999/105/EC of 22 December 1999 on the marketing of forest reproductive material. Off J Eur Union. 1999;L240:34–8.
OECD scheme for the certification of forest reproductive material moving in international trade. Paris: Organization for Economic Cooperation and Development Trade and Agriculture Directorate. 2022
FAO. The internet trade (e-commerce) in plants. Potential phytosanitary risks. Rome: IPPC, FAO; 2012.
FAO. Recommendation on: internet trade (e-commerce) in plants and other regulated articles. Rome: IPPC, FAO; 2017.
Nathan R, Schurr FM, Spiegel O, Steinitz O, Trakhtenbrot A, Tsoar A. Mechanisms of long-distance seed dispersal. Trends Ecol Evol. 2008;23:638–47.
International Trade Centre. Trade map [Internet]. 2023. https://www.trademap.org/Index.aspx. Accessed 16 Jan 2023.
Breitwieser F, Gandrud C, Allaire JJ, Russell KBM sankeyD3: D3 JavaScript sankey graphs from R. GitHub. 2017. https://github.com/fbreitwieser/sankeyD3. Accessed 19 Apr 2023.
Dalmazzone S, Giaccaria S. Economic drivers of biological invasions: a worldwide, bio-geographic analysis. Ecol Econ. 2014;105:154–65.
Liebhold AM, Brockerhoff EG, Kimberley M. Depletion of heterogeneous source species pools predicts future invasion rates. J Appl Ecol. 2017;54:1968–77.
Decourcelle T, Piou D, Desprez-Loustau ML. Detection of Diplodia sapinea in Corsican pine seeds. Plant Pathol. 2015;64:442–9.
Talgø V, Brodal G, Klemsdal SS, Stensvand A. Seed borne fungi on Abies spp. Seed Sci Technol. 2010;38:477–93.
Eurostat. Globalisation patterns in EU trade and investment. (2022) https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Globalisation_patterns_in_EU_trade_and_investment. Accessed 12 June 2023.
Gosling P. Raising trees and shrubs from seed. UK: Forestry Commission; 2007.
Epners Z. A new psychrophilic fungus causing germination failure of conifer seeds. Can J Bot. 1964;42:1589–604.
Salerno BMI, Lori GA. Association of seed-borne Fusarium species on Pinus ponderosa with germination and seedling viability in Argentina. For Pathol. 2007;37:263–71.
Carey BWA, Oak SW, Enebak SA. Pitch canker ratings of longleaf pine clones correlate with Fusarium circinatum infestation of seeds and seedling mortality in containers. For Pathol. 2005;35:205–12.
Sutherland JR, Lock W, Farris SH. Sirococcus blight: a seed-borne disease of container-grown spruce seedlings in Coastal British Columbia forest nurseries. Can J Bot. 1981;59:559–62.
Axelrood PE, Neumann M, Trotter D, Radley R, Shrimpton G, Dennis J. Seedborne Fusarium on Douglas-fir: pathogenicity and seed stratification method to decrease Fusarium contamination. New For. 1995;9:35–51.
Storer AJ, Gordon TR, Clark SL. Association of the pitch canker fungus, Fusarium subglutinans f.sp. pini, with Monterey pine seeds and seedlings in California. Plant Pathol. 1998;47:649–56.
Evira-Recuenco M, Iturritxa E, Raposo R. Impact of seed transmission on the infection and development of pitch canker disease in Pinus radiata. Forests. 2015;6:3353–68.
Bihon W, Slippers B, Burgess T, Wingfield MJ, Wingfield BD. Sources of Diplodia pinea endophytic infections in Pinus patula and P. radiata seedlings in South Africa. For Pathol. 2011;41:370–5.
Spiers AG, Wenham HT. Poplar seed transmission of Marssonina brunnea. Eur J For Pathol. 1983;13:305–14.
Newcombe G, Harding A, Ridout M, Busby PE. A hypothetical bottleneck in the plant microbiome. Front Microbiol. 2018;9:1645.
Khare E, Mishra J, Arora NK. Multifaceted interactions between endophytes and plant: developments and prospects. Front Microbiol. 2018;9:1–12.
Otten W, Gilligan CA. Soil structure and soil-borne diseases: Using epidemiological concepts to scale from fungal spread to plant epidemics. Eur J Soil Sci. 2006;57:26–37.
Termorshuizen AJ, Jeger MJ. Strategies of soilborne plant pathogenic fungi in relation to disease suppression. Fungal Ecol. 2008;1:108–14.
Dvořák M, Janoš P, Botella L, Rotková G, Zas R. Spore dispersal patterns of Fusarium circinatum on an infested monterey pine forest in North-Western Spain. Forests. 2017;8:432.
EFSA (European Food Safety Authority), Kinkar M, Vos S. Pest survey card on Fusarium circinatum. EFSA Support Publ. 2020; EN–1842.
Fabre B, Piou D, Desprez-Loustau ML, Marçais B. Can the emergence of pine Diplodia shoot blight in France be explained by changes in pathogen pressure linked to climate change? Glob Chang Biol. 2011;17:3218–27.
Wall RE, Magasi LP. Environmental factors affecting Sirococcus shoot blight of black spruce. Can J For Res. 1976;6:448–52.
• Fort T, Pauvert C, Zanne AE, Ovaskainen O, Caignard T, Barret M, et al. Maternal effects shape the seed mycobiome in Quercus petraea. New Phytol. 2021;230:1594–608. Paper demonstrates that maternal effects, environmental filtering and biotic interactions all shape the seed mycobiome of sessile oak Quercus petraea.
Parker IM, Gilbert GS. The evolutionary ecology of novel plant-pathogen interactions. Annu Rev Ecol Evol Syst. 2004;35:675–700.
Thines M. An evolutionary framework for host shifts – jumping ships for survival. New Phytol. 2019;224:605–17.
Prospero S, Cleary M. Effects of host variability on the spread of invasive forest diseases. Forests. 2017;8:1–21.
Ghelardini L, Luchi N, Pecori F, Pepori AL, Danti R, Della Rocca G, et al. Ecology of invasive forest pathogens. Biol Invasions. 2017;19:3183–200.
Laubray S, Buée M, Marçais B. Evidence of a component Allee effect for an invasive pathogen: Hymenoscyphus fraxineus, the ash dieback agent. Biol Invasions. 2023;25(8):1–16.
Paap T, Wingfield MJ, Burgess TI, Wilson JRU, Richardson DM, Santini A. Invasion frameworks: a forest pathogen perspective. Curr For Reports. 2022;8:74–89.
Wingfield MJ, Slippers B, Roux J, Wingfield BD. Worldwide movement of exotic forest fungi, especially in the tropics and the southern Hemisphere. Bioscience. 2001;51:134–40.
Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarošík V, et al. A proposed unified framework for biological invasions. Trends Ecol Evol. 2011;26:333–9.
Stanosz GR, Blodgett JT, Smith DR, Kruger EL. Water stress and Sphaeropsis sapinea as a latent pathogen of red pine seedlings. New Phytol. 2001;149:531–8.
Hanso M, Drenkhan R. Diplodia pinea is a new pathogen on Austrian pine (Pinus nigra) in Estonia. Plant Pathol. 2009;58:797.
Oliva J, Boberg J, Stenlid J. First report of Sphaeropsis sapinea on Scots pine (Pinus sylvestris) and Austrian pine (P. nigra) in Sweden. New Dis Reports. 2013;27:23–3.
CABI. Seiridium cardinale (cypress canker). UK: CABI Compend; 2021.
Battisti A, Roques A, Colombari F, Frigimelica G, Guido M. Efficient transmission of an introduced pathogen via an ancient insect-fungus association. Naturwissenschaften. 1999;86:479–83.
Luchi N, Mancini V, Feducci M, Santini A, Capretti P. Leptoglossus occidentalis and Diplodia pinea: a new insect-fungus association in Mediterranean forests. For Pathol. 2012;42:246–51.
Hoover K, Wood DL, Storer AJ, Fox JW, Bros WE. Transmission of the pitch canker fungus, Fusarium subglutinans f. sp. pini, to Monterey pine, Pinus radiata, by cone- and twig-infesting beetles. Can Entomol. 1996;128:981–94.
Zamora-Ballesteros C, Diez JJ, Martín-García J, Witzell J, Solla A, Ahumada R, et al. Pine pitch canker (PPC): pathways of pathogen spread and preventive measures. Forests. 2019;10:1–25.
Jaynes R, DePalma N. Natural infection of nuts of Castanea dentata by Endothia parasitica. Phytopathology. 1984;74:296–9.
Bragança H, Diogo E, Moniz F, Amaro P. First report of pitch canker on pines caused by Fusarium circinatum in Portugal. Plant Dis. 2009;93:1079.
FAO. ISPM 27. Diagnostic protocols for regulated pests DP 22: Fusarium circinatum. Rome: IPPC, FAO; 2017.
Ganley RJ, Watt MS, Manning L, Iturritxa E. A global climatic risk assessment of pitch canker disease. Can J For Res. 2009;39:2246–56.
Skulason B, Hansen OK, Thomsen IM, Talgø V, Nielsen UB. Damage by Neonectria neomacrospora and Adelges piceae in provenance trials of subalpine fir (Abies lasiocarpa) in Denmark. For Pathol. 2017;47:1–12.
Danti R, Barberini S, Pecchioli A, Di Lonardo V, Della RG. The epidemic spread of Seiridium cardinale on Leyland cypress severely limits its use in the Mediterranean. Plant Dis. 2014;98:1081–7.
Graniti A. Cypress canker: a pandemic in progress. Annu Rev Phytopathol. 1998;36:91–114.
EU. Regulation 2016/2031 of the European Parliament and the Council on protective measures against pests of plants. Off J Eur Union. 2016;L317:4–104.
EU. Commission Implementing Regulation (EU) 2019/2072 of the European Parliament and the Council on establishing uniform conditions for the implementation of Regulation (EU) 2016/2031. Off J Eur Union. 2019;L319:1–279.
FAO. ISPM 7. Phytosanitary certification system. Rome: IPPC, FAO; 2016.
Cram MM, Fraedrich SW. Seed diseases and seedborne pathogens of North America. Tree Plant Notes. 2009;53:35–44.
Liebhold AM, Brockerhoff EG, Garrett LJ, Parke JL, Britton KO. Live plant imports: the major pathway for forest insect and pathogen invasions of the US. Front Ecol Environ. 2012;10:135–43.
EPPO. PM 7/91 (2) Fusarium circinatum (formerly Gibberella circinata). EPPO Bull. 2019;49:228–47.
FAO. ISPM 31. Methodologies for sampling consignments. Rome: IPPC, FAO; 2008.
ISTA. 7–009: Detection of Fusarium circinatum in Pinus spp. (pine) and Pseudotsuga menziesii (Douglas fir) seed. Switzerland: The International Seed Testing Association (ISTA); 2023.
Ioos R, Fourrier C, Iancu G, Gordon TR. Sensitive detection of Fusarium circinatum in pine seed by combining an enrichment procedure with a real-time polymerase chain reaction using dual-labeled probe chemistry. Phytopathology. 2009;99:582–90.
FAO. Preparing to use high-throughput sequencing (HTS) technologies as a diagnostic tool for phytosanitary purposes. Commission on Phytosanitary Measures Recommendation No. 8. Rome: IPPC, FAO; 2019.
Nilsson RH, Anslan S, Bahram M, Wurzbacher C, Baldrian P, Tedersoo L. Mycobiome diversity: high-throughput sequencing and identification of fungi. Nat Rev Microbiol. 2019;17:95–109.
• Oskay F, Vettraino AM, Doğmuş HT, Lehtijärvi A, Woodward S, Cleary M. Seed quantity affects the fungal community composition detected using metabarcoding. Sci Rep. 2022;12:1–10. The authors examined how increasing sample size influences diversity of fungal communities detected by HTS in Pinus sylvestris seeds. The results showed that fungal alpha diversity increased with sample size. Also, community composition was significantly different between samples of different sample size.
FAO. ISPM 11. Pest risk analysis for quarantine pests. Rome: IPPC, FAO; 2017.
Cram MM. Damping-off. Tree Planters’ Notes. 2003;50(1):9–13.
Australian Government. Seed for sowing products. Canberra, Australia: Department of agriculture, water and the environment (DAWE); 2022.
Ministry for Primary Industries (MPI). Treatment requirement: approved biosecurity treatments. New Zealand: Ministry for Primary Industries; 2018.
Rees AA, Phillips DH. Detection, presence and control of seed- borne pests and and diseases of trees with special reference to seeds of tropical and sub-tropical pines. Danida Forest Seed Centre; 1986.
Martín-García J, Zas R, Solla A, Woodward S, Hantula J, Vainio EJ, et al. Environmentally friendly methods for controlling pine pitch canker. Plant Pathol. 2019;68:843–60.
Jeong RD, Shin EJ, Chu EH, Park HJ. Effects of ionizing radiation on postharvest fungal pathogens. Plant Pathol J. 2015;31:176–80.
Songsri P, Surinam B, Sanitchon J, Srisawangwong S, Kesmala T. Effects of gamma radiation on germination and growth characteristics of physic nut (Jatropha curcas L.). Aust J Biol Sci. 2011;11:268–74.
Thapa CB. Effect of acute exposure of gamma rays on seed germination and seedling growth of Pinus kesiya Gord and P. wallichiana A.B. Jacks Our Nat. 2004;2:13–7.
Ramsfield TD, Ball RD, Gardner JF, Dick MA. Temperature and time combinations required to cause mortality of a range of fungi colonizing wood. Can J Plant Pathol. 2010;32:368–75.
Núñez MR, Calvo L. Effect of high temperatures on seed germination of Pinus sylvestris and Pinus halepensis. For Ecol Manage. 2000;131:183–90.
Agustí-Brisach C, Pérez-Sierra A, Armengol J, García-Jiménez J, Berbegal M. Efficacy of hot water treatment to reduce the incidence of Fusarium circinatum on Pinus radiata seeds. Forestry. 2012;85:629–35.
Berbegal M, Landeras E, Sánchez D, Abad-Campos P, Pérez-Sierra A, Armengol J. Evaluation of Pinus radiata seed treatments to control Fusarium circinatum: effects on seed emergence and disease incidence. For Pathol. 2015;45:525–33.
Iturritxa E, Desprez-Loustau ML, García-Serna I, Quintana E, Mesanza N, Aitken J. Effect of alternative disinfection treatments against fungal canker in seeds of Pinus radiata. Seed Technol. 2011;33:88–110.
Berg G, Raaijmakers JM. Saving seed microbiomes. ISME J. 2018;12:1167–70.
Acknowledgements
We would like to thank the EUPHRESCO A-339 COSEPATH project: “Seed-borne pathogens of conifers”, part of the EUPHRESCO network, for the opportunity for scientist collaboration. We would also like to thank Gerard Clover (Centre for Forest Protection, Forest Research, UK) for critically reviewing the manuscript prior to submission.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL. Iva Franić was funded by the Swiss National Science Foundation Grant number P500PB_211040.
This study was supported by Partnerskap Alnarp Project 1407, SLU Forest Damage Centre and Kungl. Skogs-och Lantbruksakademien KSLA Grant CF2022-0022.
Helena Bragança was supported by INIAV IP and FCT (Portugal) through the R&D Unit “GREEN-IT-Bioresources for Sustainability” (UIDB/04551/2020 and UIDP/04551/2020) and LS4FUTURE Associated Laboratory (LA/P/0087/2020).
Guro Brodal and Venche Talgø were funded by the Norwegian Institute of Bioeconomy Research (NIBIO).
Anne Chandelier was funded by the Belgian Federal Service Health, Food Chain and Environment (project RF21/6344 ALERTSEED).
Thomas L. Cech and Katharina Schwanda were financially supported by the Austrian Federal Ministry of Agriculture, Forestry, Regions and Water Management (BML), Stubenring 1, 1010 Vienna, Austria.
René Eschen was supported by CABI. CABI is an international intergovernmental organisation, and we gratefully acknowledge the core financial support from our member countries (and lead agencies) including the UK (Foreign, Commonwealth & Development Office), China (Chinese Ministry of Agriculture and Rural Affairs), Australia (Australian Centre for International Agricultural Research), Canada (Agriculture and Agri-Food Canada), Netherlands (Directorate-General for International Cooperation) and Switzerland (Swiss Agency for Development and Cooperation). See https:// www. cabi. org/ about- cabi/ who- we- work- with/ key- donors/ for full details.
This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions neither the European Union nor the European Commission can be considered responsible for them.
Ana Pérez-Sierra was funded by the Department for Environment, Food & Rural Affairs (DEFRA) and Forest Research (UK).
Author information
Authors and Affiliations
Contributions
Manuscript idea was conceived by I.F. and developed in the discussion with all co-authors. First draft of the manuscript was written by all co-authors under supervision of I.F. and A.P-S. with contributions as follows: I.F. and A.P-S. (Introduction and Conclusions); M.C., K.Si. and M.T. (Transport of pathogens into a new country); G.B., T.D-L., A.L., A.G.A.K. and V.T. (Introduction of pathogens into a new environment); A.C., T.L.C. and K.Sc. (Establishment of pathogens in the new environment); S.P., H.B. and H.S. (Spread of pathogens in a new environment); M.O., R.E. and AM.V. (Phytosanitary Management of Tree Seeds). I.F., A.P-S and S.P. reviewed and edited the manuscript draft and prepared the final version of the manuscript. Data collection for the analysis of the tree seed trade was done by M.O. and analysis was conducted by M.O., I.F. and R.E.. Figures were created by A.P-S (Fig. 1), S.P (Fig. 2) and I.F. (Fig. 3).
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franić, I., Cleary, M., Aday Kaya, A.G. et al. The Biosecurity Risks of International Forest Tree Seed Movements. Curr. For. Rep. 10, 89–102 (2024). https://doi.org/10.1007/s40725-023-00211-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40725-023-00211-3