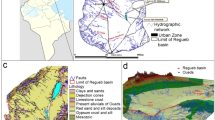
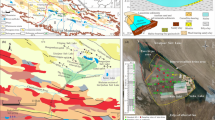
Abstract
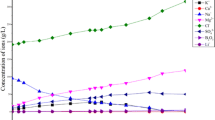
The purpose of this study is to examine hydrochemical changes of saline groundwater and precipitation of mineral phases during evaporative graduation processes. Therefore, concentrated brines and mineral precipitates were sampled at four graduation towers with differing evaporation approaches. Solid phase compositions were qualitatively and quantitatively analysed. Hydrochemistry changes of dissolved ion concentrations during successive evaporation was assessed by comparing natural with concentrated brines. PHREEQC was used to simulate sequence and quantity of mineral precipitates. Finally, a prognosis for expected precipitations of a newly built graduation tower was made using PHREEQC modelling. The identified mineral phases included calcite, aragonite, gypsum and halite in varying proportions. Concentrations of all investigated ions (except bicarbonate) increase, eventually leading to supersaturation and sequential precipitation of evaporite minerals. Modelled saturation indices show that calcite is the first and halite is one of the last precipitating phases at all sites. Further calculated precipitates include the carbonates dolomite, siderite and strontianite; manganese oxide and hematite; and the sulphates baryte, celestine and gypsum which precipitate depending on local hydrogeochemistry and graduation conditions. Calculated precipitation quantities reach a maximum of 48.6 g/L of applied natural brine at an evaporation grade of 90%. After 25 years, the total expected mass of precipitates at the new graduation tower is about 34.4 t for a graduation process up to a salt content of 19%, and about 356 t for a permanent evaporation grade of 90%.
Highlights
• Except for bicarbonate, concentrations of all ions increase to different degrees.
• Mineral precipitations are composed of calcite, aragonite, gypsum and halite.
• Modelled precipitation sequence: carbonates, sulphates, chlorides.
• Modelled precipitation amounts: max. 48.6 g/L for natural brine at 90% evaporation.
• 25 y precipitation forecast for new graduation tower: max. 356 t at 90% evaporation.




Similar content being viewed by others
Data Availability
on request.
Code Availability
PHREEQC, publicly available code for hydrogeochemical calculations: https://www.usgs.gov/software/phreeqc-version-3
References
AD-HOC-AG Hydrogeologie (2016) Regionale Hydrogeologie von Deutschland – Die Grundwasserleiter: Verbreitung, Gesteine, Lagerungsverhältnisse, Schutz und Bedeutung. [Regional hydrogeology of Germany]. Geol Jb A, 163:456 p, Hannover [in German]
Ball JW, Nordstrom DK (1991) WATEQ4F – User’s manual with revised thermodynamic data base and test cases for calculating speciation of major, trace and redox elements in natural waters. U.S. Geological Survey Open-File Report 91–183:193p, Menlo Park, CA. https://doi.org/10.3133/ofr91183
Banning A, Rüde TR, Dölling B (2013) Crossing redox boundaries-aquifer redox history and effects on iron mineralogy and arsenic availability. J Haz Mat 262:905–914. https://doi.org/10.1016/j.jhazmat.2012.12.015
Bredensteiner O (2020) Fakten zum neuen Gradierwerk [Facts of the new graduation tower]. inwebco GmbH. [in German]. https://www.badsassendorf.de/de/Erleben-Geniessen/Kurpark/Fakten-zum-neuen-Gradierwerk. Accessed 28 June 2020
Burkowska-But A, Kalwasińska A, Brzezinska M (2014) The role of open-air inhalatoria in the air quality improvement in spa towns. Int J Occup Med Environ Health 27(4):560–570. https://doi.org/10.2478/s13382-014-0274-8
Chruszcz-Lipska K, Winid B, Madalska GA, Macuda J, Łukanko Ł (2021) High content of boron in curative water: from the spa to industrial recovery of borates? (Poland as a case study). Minerals 11:8. https://doi.org/10.3390/min11010008
Elsner H (2016) Salze in Deutschland [Salts in Germany]. 103 p, Bundesanstalt für Geowissenschaften und Rohstoffe, Hannover [in German]
Engelhardt H-J (2015) Die Gipskristalle des Gradierwerks Bad Kösen [Gypsum crystals of the graduation tower Bad Kösen]. Der Aufschluss 66:262–267 [in German]
Forbert S (2017) Gradierwerk: Weiter Suche nach Methode, um Dornstein zu entfernen [The search for a method to remove thornstone continues]. Verlag Dierichs [in German] https://www.hna.de/lokales/witzenhausen/bad-sooden-allendorf-ort83103/gradierwerk-weiter-suche-nach-methode-dornstein-entfernen-7435326.html. Accessed 18 April 2020
Führer D (2020) Klima Bad Sassendorf - Station Lippstadt-Bökenförde (92 m) [Climate Bad Sassendorf]. Wetterdienst.de. https://www.wetterdienst.de/Deutschlandwetter/ Bad_Sassendorf/Klima/. Accessed 7 July 2020
Geologischer Dienst Nordrhein-Westfalen (GD NRW) (2016) Geologie und Boden in Nordrhein-Westfalen [Geology and soil in Northrhine-Westphalia]. 157 p, Geologischer Dienst Nordrhein-Westfalen – Landesbetrieb, Krefeld; ISBN: 978–3–86029–938–8 [in German]
Geologisches Landesamt Nordrhein-Westfalen (GLA NRW) (1995) Geologie im Münsterland [Geology of the Münsterland]. Geologisches Landesamt Nordrhein-Westfalen (GLA), Krefeld [in German]
Grobe M, Machel HG (2002) Saline groundwater in the Münsterland Cretaceous Basin, Germany: clues to its origin and evolution. Marine Petrol Geol 19:307–322. https://doi.org/10.1016/S0264-8172(02)00019-3
Gugnacka-Fiedor W (2011) Taxonomic diversity of mosses in the area of graduation towers in the town of Ciechocinek. Ecol Questions 14:31–33. https://doi.org/10.2478/v10090-011-0008-5
Halaj E (2015) Geothermal bathing and recreation centres in Poland. Environ Earth Sci 74:7497–7509. https://doi.org/10.1007/s12665-014-3740-5
Herrmann AG, Knake D, Schneider J, Peters H (1973) Geochemistry of modern seawater and brines from salt pans: Main components and bromine distribution. Contrib Mineral Petrol 40:1–24. https://doi.org/10.1007/BF00371760
Himstedt F, Hintz E, Grünhut L, Jacobj C, Kauffmann H, Keilhack K, Kionka H, Kraus F, Kremser V, Nicolas P, Paul T, Röchling F, Scherrer A, Schütze C, Winckler A, Rost E, Sonntag G, Auerbach F (1907) Deutsches Bäderbuch [Manual of spa therapy and German Spas]. Weber, Leipzig [in German]
Jones B (2017) Review of calcium carbonate polymorph precipitation in spring systems. Sediment Geol 353:64–75. https://doi.org/10.1016/j.sedgeo.2017.03.006
Kalwasińska A, Deja-Sikora E, Burkowska-But A, Szabó A, Felföldi T, Kosobucki P, Krawiec A, Walczak M (2018) Changes in bacterial and archaeal communities during the concentration of brine at the graduation towers in Ciechocinek spa (Poland). Extremophiles 22:233–246. https://doi.org/10.1007/s00792-017-0992-5
Kaseb HE, Torshizian HA, Jahani D, Javanbakht M, Ghadimvand MK (2020) Effects of lithological and evolutionary processes on geochemical changes of Shahrokht-Yazdan Playa brines (east of Iran-west of Afghanistan). Arab J Geosci 13:1070. https://doi.org/10.1007/s12517-020-05897-4
Käß W, Käß H (2008) Deutsches Bäderbuch, 2. Aufl. [Manual of spa therapy and German Spas, 2nd edn.]. Schweizerbart, Stuttgart [in German]
Krzyzaniak-Sitarz M (2012) Influence of graduation towers on average annual cations content in black earths (mollic gleysols) in the Inowroclaw city. Ecol Chem Engin A 19(6):573–582. https://doi.org/10.2428/ecea.2012.19(06)058
Martínez-Pillado V, Yusta I, Iriarte E, Álvaro A, Ortega N, Aranburu A, Arsuaga JL (2020) The red coloration of Goikoetxe Cave’s speleothems (Busturia, Spain): an indicator of paleoclimatic changes. Quaternary Internat 566–567:141–151. https://doi.org/10.1016/j.quaint.2020.04.006
Merkel BJ, Planer-Friedrich B (2008) Grundwasserchemie – Praxisorientierter Leitfaden zur numerischen Modellierung von Beschaffenheit, Kontamination und Sanierung aquatischer Systeme [Groundwater chemistry]. Springer, Berlin [in German]
Nasri N, Bouhlila R, Saaltink MW, Gamazo P (2015) Modeling the hydrogeochemical evolution of brine in saline systems: case study of the Sabkha of Oum El Khialate in South East Tunisia. Appl Geochem 55:160–169. https://doi.org/10.1016/j.apgeochem.2014.11.003
Nienartowicz A, Piernik A, Marcykiewicz J, Lis M, Kunz M, Viglia S (2011) Halophytes as components of lawns under different soil salinity and human impact in the health resort “Ciechocinek.” Ecol Quest 14:21–24. https://doi.org/10.12775/v10090-011-0006-7
Parkhurst DL, Appelo CAJ (2013) Description of Input and Examples for PHREEQC Version 3-A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. U.S. Geological Survey Techniques and Methods, book 6, chap. A43, 497 p. http://pubs.usgs.gov/tm/06/a43/. Accessed 20 April 2020
Petschick R (2002) Röntgendiffraktometrie in der Sedimentologie (K5) [X-ray diffractometry in sedimentology]. In: Hüssner H, Hinderer M, Götz A, Petschick R (eds.) Sediment. Schriften Dt Geol Ges. 18:99–118 [in German]
Pfenning GmbH (2014) Bad Dürkheim bringt Bachlauf ans Tageslicht. THIS Magazin 04/2014. https://www.thismagazin.de/artikel/tis_Bad_Duerkheim_bringt_Bachlauf_ans_Tageslicht_1991275. Accessed 3 Feb 2021
Pitzer KS (1973) Thermodynamics of electrolytes – 1 Theoretical basis and general equations. J Phys Chem. 77(2):268–277. https://doi.org/10.1021/j100621a026
Plummer LN, Parkhurst DL, Fleming GW, Dunkle SA (1988) A computer program incorporating Pitzer’s equations for calculation of geochemical reactions in brines. U.S. Geological Survey Water-Resources Investigations Report 88–4153:313p, Reston, VA. https://doi.org/10.3133/wri884153
Putz H (2019) Match! - Phase Identification from Powder Diffraction, Crystal Impact - Dr. H. Putz & Dr. K. Brandenburg GbR, Bonn, Germany. https://www.crystalimpact.de/match. Accessed 25 July 2019
Reznik IJ, Gavrieli I, Ganor J (2009) Kinetics of gypsum nucleation and crystal growth from Dead Sea brine. Geochim Cosmochim Acta 73:6218–6230. https://doi.org/10.1016/j.gca.2009.07.018
Roth J (1879) Allgemeine und chemische Geologie - Bildung und Umbildung der Mineralien. Quell-, Fluss- und Meerwasser; Die Absätze [General and chemical geology – formation and transformation of minerals. Springs, rivers and sea water; precipitations]. Hertz, Berlin [in German]
Schäffer R, Sass I (2016) Ausbreitung und Vermischung geogener, kohlendioxidführender Thermalsole in oberfächennahem Grundwasser, Bad Nauheim [Migration and mixing of a carbon dioxide bearing thermal brine in shallow aquifers, Bad Nauheim, Germany]. Grundwasser 21:305–319. https://doi.org/10.1007/s00767-016-0341-0 [in German]
Schäffer R (2018) Hydrochemische Methoden zur geothermalen Erkundung und Charakterisierung von Thermalwässern [Hydrochemical methods for geothermal exploration and characterization of thermal waters]. Dissertation, Technische Universität Darmstadt, URN: urn:nbn:de:tuda-tuprints-75024 [in German]
Schäffer R, Bär K, Sass I (2018) Multimethod exploration of the hydrothermal reservoir in Bad Soden-Salmünster, Germany. Z Dt Ges Geowiss 169:311–333. https://doi.org/10.1127/zdgg/2018/0147
Scholz F, Kahlert H (2018) Chemische Gleichgewichte in der Analytischen Chemie – Die Theorie der Säure-Base-, Komplexbildungs-, Fällungs- und Redoxgleichgewichte [Chemical equilibria in analytical chemistry]. Springer, Berlin [in German]
Schulze-Seeger W (1988) Von der Salzstadt zum Heilbad – Beiträge zur Salinen- und Ortsgeschichte von Bad Orb [Bad Orb – from a salt town to a spa town]. Orbensien Edmund Acker, Bad Orb [in German]
Stockmann C, Stockmann A (1998) Die Saline ,,Gottesgabe" in Rheine - Ein Beitrag zur Salzgewinnung und Salzvermarktung in Westfalen [Saline „Gottesgabe“ in Rheine – about salt production and salt marketing in Westphalia]. Geographische Kommission für Westfalen, 15, Münster [in German]
Wisotzky F, Cremer N, Lenk S (2018) Angewandte Grundwasserchemie, Hydrogeologie und hydrogeochemische Modellierung – Grundlagen, Anwendungen und Problemlösungen [Applied groundwater chemistry, hydrogeology and hydrogeochemical modelling]. Springer, Berlin [in German]
Acknowledgements
The authors thank the contact persons of the graduation towers: Mr. Vleugels (Rheine), Mr. Meck (Bad Sassendorf), Mr. Köchling and Mr. Kemper (Bad Westernkotten) as well as Mr. Wilker (Bad Rothenfelde) and the Kurverwaltung Bad Rothenfelde GmbH for enabling the sampling as well as for providing data for the natural brines. Our thanks also go to Ilka Hinzer (RUB) and Nikolaus Richard (RUB) for carrying out hydrochemical analyses and to Hartmut Mammen (RUB) for carrying out powder X-ray diffraction measurements.
Author information
Authors and Affiliations
Contributions
Conceptualization: Andre Banning, Laura-Jane Ostwald; Methodology: Andre Banning, Laura-Jane Ostwald; Formal analysis and investigation: Laura-Jane Ostwald; Writing—original draft preparation: Laura-Jane Ostwald; Writing—review and editing: Andre Banning; Resources: Andre Banning; Supervision: Andre Banning.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
the authors declare that there is no conflict of interest or competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ostwald, LJ., Banning, A. Mineral Precipitations and Hydrochemical Evolution of Brines in Graduation Towers. Environ. Process. 8, 729–746 (2021). https://doi.org/10.1007/s40710-021-00511-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-021-00511-5