Opinion statement
Purpose of Review This review discusses the epidemiology, evaluation and treatment of overlap inflammatory myopathy syndromes.
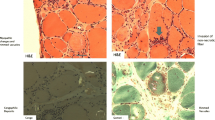
Recent findings Evaluation of overlap myopathy in systemic sclerosis (SSc) has shown that the disease is often heterogeneous with variable presentation, histopathology, and response to therapy. These differences have shown to be important in management, specifically with the response to immunosuppression. Inflammation and necrosis are features suggestive of a positive response, whereas fibrosis and neurogenic atrophy are also common and may be less responsive to therapy.
Summary Inflammatory myopathy associated with overlap connective tissue disease is often associated with greater morbidity, and there are few studies to guide treatment. Treatment is often tailored to the patient based on severity of muscular and concomitant extramuscular disease. While further research is needed to better understand treatment of overlap syndromes, the absence of consensus criteria remains a limiting factor.
Similar content being viewed by others
References and Recommended Reading
Recently published papers of particular interest have been highlighted as: • Of importance •• Of major importance
Váncsa A, Gergely L, Ponyi A, et al. Myositis-specific and myositis-associated antibodies in overlap myositis in comparison to primary dermatopolymyositis: relevance for clinical classification: retrospective study of 169 patients. Jt Bone Spine Rev Rhum. 2010;77(2):125–30.
Leclair V, Regardt M, Wojcik S, et al. Health-related quality of life (HRQoL) in idiopathic inflammatory myopathy: a systematic review. PLoS One. 2016;11(8):e0160753.
Feldon M, Farhadi PN, Brunner HI, et al. Predictors of reduced health-related quality of life in adult patients with idiopathic inflammatory myopathies. Arthritis Care Res. 2017; https://doi.org/10.1002/acr.23198.
Regardt M, Welin Henriksson E, Sandqvist J, et al. Work ability in patients with polymyositis and dermatomyositis: an explorative and descriptive study. Work. 2015;53(2):265–77.
Bradford Rice J, White A, Lopez A, et al. Healthcare resource utilization and work loss in dermatomyositis and polymyositis patients in a privately-insured US population. J Med Econ. 2016;19(7):649–54.
Paik JJ, Wigley FM, Mejia AF, et al. Independent association of severity of muscle weakness with disability as measured by the health assessment questionnaire disability index in scleroderma. Arthritis Care Res (Hoboken). 2016;68(11):1695–703.
Paik JJ. Myopathy in scleroderma and in other connective tissue diseases. Curr Opin Rheumatol. 2016;28(6):631–5.
West SG, Killian PJ, Lawless OJ. Association of myositis and myocarditis in progressive systemic sclerosis. Arthritis Rheum. 1981;24(5):662–8.
Medsger TA, Rodnan GP, Moossy J, et al. Skeletal muscle involvement in progressive systemic sclerosis (scleroderma). Arthritis Rheum. 1968;11(4):554–68.
Clements PJ, Furst DE, Campion DS, et al. Muscle disease in progressive systemic sclerosis: diagnostic and therapeutic considerations. Arthritis Rheum. 1978;21(1):62–71.
Mimori T. Scleroderma-polymyositis overlap syndrome. clinical and serologic aspects. Int J Dermatol. 1987;26(7):419–25.
Garcin B, Lenglet T, Dubourg O, et al. Dropped head syndrome as a presenting sign of scleromyositis. J Neurol Sci. 2010;292(1–2):101–3.
Jung M, Bonner A, Hudson M, et al. Myopathy is a poor prognostic feature in systemic sclerosis: results from the Canadian Scleroderma Research Group (CSRG) cohort. Scand J Rheumatol. 2014;43(3):217–20.
Bhansing KJ, Lammens M, Knaapen HK, et al. Scleroderma-polymyositis overlap syndrome versus idiopathic polymyositis and systemic sclerosis: a descriptive study on clinical features and myopathology. Arthritis Res Ther. 2014;16(3):R111.
• Ranque B, Authier FJ, Le-Guern V, et al. A descriptive and prognostic study of systemic sclerosis-associated myopathies. Ann Rheum Dis. 2009;68(9):1474–7. This study suggests that the response to immunosuppression is related to presence of inflammation on biopsy
Paik JJ, Wigley FM, Shah AA, et al. Fibrosing myopathy in systemic sclerosis associates with higher mortality. Arthritis Care Res (Hoboken). 2017; https://doi.org/10.1002/acr.23291.
Toledano C, Gain M, Kettaneh A, et al. Aldolase predicts subsequent myopathy occurrence in systemic sclerosis. Arthritis Res Ther. 2012;14(3):R152.
Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35(1):35–42.
Bell S, Krieg T, Meurer M. Antibodies to Ro/SSA detected by ELISA: correlation with clinical features in systemic scleroderma. Br J Dermatol. 1989;121(1):35–41.
Koschik RW, Fertig N, Lucas MR, et al. Anti-PM-Scl antibody in patients with systemic sclerosis. Clin Exp Rheumatol. 2012;30(2 Suppl 71):S12–6.
Vandergheynst F, Ocmant A, Sordet C, et al. Anti-pm/scl antibodies in connective tissue disease: clinical and biological assessment of 14 patients. Clin Exp Rheumatol. 2006;24(2):129–33.
•• Paik JJ, Wigley FM, Lloyd TE, et al. Spectrum of muscle histopathologic findings in forty-two scleroderma patients with weakness. Arthritis Care Res (Hoboken). 2015;67(10):1416–25. This study provides histopathologic findings in patients with scleroderma and weakness showing the high prevalence of neurogic atrophy and fibrosis
Schanz S, Henes J, Ulmer A, et al. Magnetic resonance imaging findings in patients with systemic scleroderma and musculoskeletal symptoms. Eur Radiol. 2013;23(1):212–21.
Ranque B, Authier FJ, Berezne A, et al. Systemic sclerosis-associated myopathy. Ann N Y Acad Sci. 2007;1108:268–82.
Steen VD, Medsger TA. Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum. 1998;41(9):1613–9.
Guillevin L, Berezne A, Seror R, et al. Scleroderma renal crisis: a retrospective multicentre study on 91 patients and 427 controls. Rheumatology (Oxford). 2012;51(3):460–7.
Ruperto N, Pistorio A, Oliveira S, et al. Prednisone versus prednisone plus ciclosporin versus prednisone plus methotrexate in new-onset juvenile dermatomyositis: a randomised trial. Lancet. 2016;387(10019):671–8.
Vencovský J, Jarosova K, Machacek S, et al. Cyclosporine A versus methotrexate in the treatment of polymyositis and dermatomyositis. Scand J Rheumatol. 2000;29(2):95–102.
Ibrahim F, Choy E, Gordon P, et al. Second-line agents in myositis: 1-year factorial trial of additional immunosuppression in patients who have partially responded to steroids. Rheumatology (Oxford). 2015;54(6):1050–5.
Majithia V, Harisdangkul V. Mycophenolate mofetil (CellCept): an alternative therapy for autoimmune inflammatory myopathy. Rheumatology (Oxford). 2005;44(3):386–9.
Mendoza FA, Nagle SJ, Lee JB, et al. A prospective observational study of mycophenolate mofetil treatment in progressive diffuse cutaneous systemic sclerosis of recent onset. J Rheumatol. 2012;39(6):1241–7.
Liossis SN, Bounas A, Andonopoulos AP. Mycophenolate mofetil as first-line treatment improves clinically evident early scleroderma lung disease. Rheumatology (Oxford). 2006;45(8):1005–8.
Dalakas MC, Illa I, Dambrosia JM, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. 1993;329(27):1993–2000.
Cherin P, Pelletier S, Teixeria A, et al. Results and long-term followup of intravenous immunoglobulin infusions in chronic, refractory polymyositis: an open study with thirty-five adult patients. Arthritis Rheum. 2002;46(2):467–74.
Saito E, Koike T, Hashimoto H, et al. Efficacy of high-dose intravenous immunoglobulin therapy in Japanese patients with steroid-resistant polymyositis and dermatomyositis. Mod Rheumatol. 2008;18(1):34–44.
Pisoni CN, Cuadrado MJ, Khamashta MA, et al. Mycophenolate mofetil treatment in resistant myositis. Rheumatology (Oxford). 2007;46(3):516–8.
Abelha-Aleixo J, Bernardo A, Costa L. Benefit of intravenous immunoglobulin in a patient with longstanding polymyositis/systemic sclerosis overlap syndrome. Acta Reumatol Port. 2015;40(2):176–8.
Mauhin W, Rivière S, Cabane J, et al. Improvement in lung fibrosis using intravenous immunoglobulin in systemic sclerosis with myositis. Scand J Rheumatol. 2014;43(2):170–1.
Poelman CL, Hummers LK, Wigley FM, et al. Intravenous immunoglobulin may be an effective therapy for refractory, active diffuse cutaneous systemic sclerosis. J Rheumatol. 2015;42(2):236–42.
Vilela VS, Maretti GB, Gama LM, et al. Rituximab for the therapy of systemic sclerosis: a series of 10 cases in a single center. Rev Bras Reumatol Engl Ed. 2016;56(5):458–63.
Fabri M, Hunzelmann N, Krieg T, et al. Discordant response to rituximab in a systemic sclerosis patient with associated myositis. J Am Acad Dermatol. 2008;58(5 Suppl 1):S127–8.
Kondo M, Murakawa Y, Matsumura T, et al. A case of overlap syndrome successfully treated with tocilizumab: a hopeful treatment strategy for refractory dermatomyositis. Rheumatology (Oxford). 2014;53(10):1907–8.
Elhai M, Meunier M, Matucci-Cerinic M, et al. Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: a EUSTAR observational study. Ann Rheum Dis. 2013;72(7):1217–20.
Miró O, Pedrol E, Casademont J, et al. Muscle involvement in rheumatoid arthritis: clinicopathological study of 21 symptomatic cases. Semin Arthritis Rheum. 1996;25(6):421–8.
Labrador-Horrillo M, Martinez MA, Selva-O’Callaghan A, et al. Anti-cyclic citrullinated peptide and anti-keratin antibodies in patients with idiopathic inflammatory myopathy. Rheumatology (Oxford). 2009;48(6):676–9.
• Aguila LA, Lopes MR, Pretti FZ, et al. Clinical and laboratory features of overlap syndromes of idiopathic inflammatory myopathies associated with systemic lupus erythematosus, systemic sclerosis, or rheumatoid arthritis. Clin Rheumatol. 2014;33(8):1093–8. This study reviews features associated with overlap syndromes
Ancuţa C, Pomirleanu DC, Anton CR, et al. Rheumatoid myositis, myth or reality? A clinical, imaging and histological study. Romanian J Morphol Embryol. 2014;55(3):781–5.
Nakajima A, Yoshino K, Soejima M, et al. High frequencies and co-existing of myositis-specific autoantibodies in patients with idiopathic inflammatory myopathies overlapped to rheumatoid arthritis. Rheumatol Int. 2012;32(7):2057–61.
Halla JT, Koopman WJ, Fallahi S, et al. Rheumatoid myositis. Clinical and histologic features and possible pathogenesis. Arthritis Rheum. 1984;27(7):737–43.
Cavagna L, Fusetti C, Montecucco C, et al. Anticyclic citrullinated peptide antibodies as markers of erosive arthritis in antisynthetase syndrome. J Rheumatol. 2010;37(9):1967.
Schmidt WA, Wetzel W, Friedlander R, et al. Clinical and serological aspects of patients with anti-Jo-1 antibodies—an evolving spectrum of disease manifestations. Clin Rheumatol. 2000;19(5):371–7.
Lefèvre G, Meyer A, Launay D, et al. Seronegative polyarthritis revealing antisynthetase syndrome: a multicentre study of 40 patients. Rheumatology (Oxford). 2015;54(5):927–32.
Nagashima T, Sato H, Minota S. Destructive arthropathy associated with dermatomyositis sine myositis positive for anti-Jo-1 and anti-cyclic citrullinated peptide antibodies. J Rheumatol. 2009;36(9):2133–4.
Meyer A, Lefevre G, Bierry G, et al. In antisynthetase syndrome, ACPA are associated with severe and erosive arthritis: an overlapping rheumatoid arthritis and antisynthetase syndrome. Medicine (Baltimore). 2015;94(20):e523.
Brunasso AM, Aberer W, Massone C. New onset of dermatomyositis/polymyositis during anti-TNF-α therapies: a systematic literature review. ScientificWorldJournal. 2014;2014:179180.
Rojas-Serrano J, Herrera-Bringas D, Perez-Roman DI, et al. Rheumatoid arthritis-related interstitial lung disease (RA-ILD): methotrexate and the severity of lung disease are associated to prognosis. Clin Rheumatol. 2017;36(7):1493–500.
Parziale N, Kovacs SC, Thomas CB, et al. Rituximab and mycophenolate combination therapy in refractory dermatomyositis with multiple autoimmune disorders. J Clin Neuromuscul Dis. 2011;13(2):63–7.
Aggarwal R, Bandos A, Reed AM. Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol. 2014;66(3):740–9.
Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65(2):314–24.
Andersson H, Sem M, Lund MB, et al. Long-term experience with rituximab in anti-synthetase syndrome-related interstitial lung disease. Rheumatology (Oxford). 2015;54(8):1420–8.
Marie I, Dominique S, Janvresse A, et al. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med. 2012;106(4):581–7.
Dayal NA, Isenberg DA. SLE/myositis overlap: are the manifestations of SLE different in overlap disease? Lupus. 2002;11(5):293–8.
Liang Y, Leng RX, Pan HF, et al. Associated variables of myositis in systemic lupus erythematosus: a cross-sectional study. Med Sci Monit. 2017;23:2543–9.
Tsokos GC, Moutsopoulos HM, Steinberg AD. Muscle involvement in systemic lupus erythematosus. JAMA. 1981;246(7):766–8.
Jakati S, Rajesekhar L, Uppin M, et al. SLE myopathy: a clinicopathological study. Int J Rheum Dis. 2015;18(8):886–91.
Torrente-Segarra V, Salman-Monte TC, Rua-Fiueroa I, et al. Fibromyalgia prevalence and related factors in a large registry of patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2016;34(2 Suppl 96):S40–7.
Lim KL, Abdul-Wahab R, Lowe J, et al. Muscle biopsy abnormalities in systemic lupus erythematosus: correlation with clinical and laboratory parameters. Ann Rheum Dis. 1994;53(3):178–82.
Nakaya I, Iwata Y, Sugiyama Y, et al. Dermatomyositis relapse complicated with gastric carcinoma and lupus nephritis five years after the initial diagnosis of dermatomyositis. Intern Med. 2002;41(6):502–3.
Kijima T, Kanekiyo S, Nakasuga C, et al. A case report of a patient with overlap syndrome systemic lupus erythematosus (SLE) and polymyositis (PM) whose condition improved following treatment for coexisting descending colon cancer. Gan To Kagaku Ryoho. 2013;40(12):1936–8.
Mascaró JM, Ferrando J, Sole MT, et al. Paraneoplastic pemphigus: a case of long-term survival associated with systemic lupus erythematosus and polymyositis. Dermatology. 1999;199(1):63–6.
Tselios K, Gladman DD, Su J, et al. Antimalarials as a risk factor for elevated muscle enzymes in systemic lupus erythematosus. Lupus. 2016;25(5):532–5.
Maazoun F, Frikha F, Snoussi M, et al. Systemic lupus erythematosusmyositis overlap syndrome: report of 6 cases. Clin Pract. 2011;1(4):e89.
Wenzel J, Uerlich M, Gerdsen R, et al. Association of inclusion body myositis with subacute cutaneous lupus erythematosus. Rheumatol Int. 2001;21(2):75–7.
Limaye V, Scott G, Kwiatek R, et al. Inclusion body myositis associated with systemic lupus erythematosus (SLE). Aust NZ J Med. 2000;30(2):275–6.
Varela-Rosario N, Pérez-Berenguer JL, Vilá LM. Efficacy of immunosuppressive treatment in a systemic lupus erythematosus patient presenting with inclusion body myositis. BMJ Case Rep. 2016;5:2016.
Stein M, Bell MJ, Ang LC. Hydroxychloroquine neuromyotoxicity. J Rheumatol. 2000;27(12):2927–31.
Meesiri S. Pyomyositis in a patient with systemic lupus erythaematosus and a review of the literature. BMJ Case Rep. 2016;2016:10.
Ravindran V, Duke O. Non-tropical pyomyositis in a patient with systemic lupus erythematosus. Lupus. 2009;18(4):379–80.
Garton MJ, Isenberg DA. Clinical features of lupus myositis versus idiopathic myositis: a review of 30 cases. Br J Rheumatol. 1997;36(10):1067–74.
Bunch TW, Worthington JW, Combs JJ, et al. Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med. 1980;92(3):365–9.
Bunch TW. Prednisone and azathioprine for polymyositis: long-term followup. Arthritis Rheum. 1981;24(1):45–8.
S L, Tony K, Raghupathy, et al. A rare case of mixed connective tissue disease (mctd) with intricate features of lupus, polymyositis and rheumatoid arthritis presenting with severe myositis. J Clin Diagn Res. 2015;9(3):OD05–7.
Wise CM, Vuyyuru S, Roberst WN. Methotrexate in nonrenal lupus and undifferentiated connective tissue disease—a review of 36 patients. J Rheumatol. 1996;23(6):1005–10.
Hashimoto M, Nonaka S, Furuta E, et al. Methotrexate for steroid-resistant systemic lupus erythematosus. Clin Rheumatol. 1994;13(2):280–3.
Luzi G, Diamanti AP, Germano V, et al. Successful treatment with intravenous immunoglobulins in a patient affected by dermatomyositis/systemic lupus erythematosus overlap syndrome and tuberculosis. Clin Immunol. 2007;125(2):127–30.
Ramos-Casals M, Garcia-Hernandez FJ, de Ramon E, et al. Off-label use of rituximab in 196 patients with severe, refractory systemic autoimmune diseases. Clin Exp Rheumatol. 2010;28(4):468–76.
Bang SY, Lee CK, Kang YM, et al. Multicenter retrospective analysis of the effectiveness and safety of rituximab in Korean patients with refractory systemic lupus erythematosus. Autoimmune Dis. 2012;2012:565039.
Aoki A, Ono S, Ueda A, et al. Myositis in primary Sjogren’s syndrome: clinical and pathological report. Mod Rheumatol. 2003;13(1):57–61.
Ramos-Casals M, Brito-Zeron P, Solans R, et al. Systemic involvement in primary Sjogren’s syndrome evaluated by the EULAR-SS disease activity index: analysis of 921 Spanish patients (GEA-SS Registry). Rheumatology (Oxford). 2014;53(2):321–31.
Lindvall B, Bengtsson A, Ernerudh J, et al. Subclinical myositis is common in primary Sjogren’s syndrome and is not related to muscle pain. J Rheumatol. 2002;29(4):717–25.
Colafrancesco S, Priori R, Gattamelata A, et al. Myositis in primary Sjogren’s syndrome: data from a multicentre cohort. Clin Exp Rheumatol. 2015;33(4):457–64.
Fauchais AL, Martel C, Gondran G, et al. Immunogogical profile in primary Sjogren syndrome: clinical significance, prognosis and long-term evolution to other autoimmune disease. Autoimmun Rev. 2010;9(9):595–9.
Vrethem M, Lindvall B, Holmgren H, et al. Neuropathy and myopathy in primary Sjogren’s syndrome: neurophysiological, immunological and muscle biopsy results. Acta Neurol Scand. 1990;82(2):126–31.
Misterska-Skora M, Sebastian A, Dziegiel P, et al. Inclusion body myositis associated with Sjogren’s syndrome. Rheumatol Int. 2013;33(12):3083–6.
Espitia-Thibault A, Masseau A, Neel A, et al. Sjogren’s syndrome-associated myositis with germinal centre-like structures. Autoimmun Rev. 2017;16(2):154–8.
Mashaly R, Hauw JJ, Poisson M, et al. Polymyositis with infiltration by lymphoid follicles. Arch Neurol. 1981;38(12):777–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
John Miller and Julie Paik declare they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Other CTD: Inflammatory Myopathies and Sjogren's
Rights and permissions
About this article
Cite this article
Miller, J.B., Paik, J.J. Overlap Syndromes in Inflammatory Myopathies. Curr Treat Options in Rheum 3, 289–298 (2017). https://doi.org/10.1007/s40674-017-0074-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40674-017-0074-y