Abstract
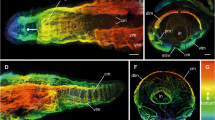
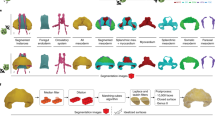
The now classical idea that programmed cell death (apoptosis) contributes to a plethora of developmental processes still has lost nothing of its impact. It is, therefore, important to establish effective three-dimensional (3D) reconstruction as well as simulation techniques to decipher the exact patterns and functions of such apoptotic events. The present study focuses on the question whether and how apoptosis promotes neurulation-associated processes in the spinal cord of Tupaia belangeri (Tupaiidae, Scandentia, Mammalia). Our 3D reconstructions demonstrate that at least two craniocaudal waves of apoptosis consecutively pass through the dorsal spinal cord. The first wave appears to be involved in neural fold fusion and/or in selection processes among premigratory neural crest cells. The second one seems to assist in establishing the dorsal signaling center known as the roof plate. In the hindbrain, in contrast, apoptosis among premigratory neural crest cells progresses craniocaudally but discontinuously, in a segment-specific manner. Unlike apoptosis in the spinal cord, these segment-specific apoptotic events, however, precede later ones that seemingly support neural fold fusion and/or postfusion remodeling. Arguing with Whitehead that biological patterns and rhythms differ in that biological rhythms depend “upon the differences involved in each exhibition of the pattern” (Whitehead in An enquiry concerning the principles of natural knowledge. Cambridge University Press, London, 1919, p. 198) we show that 3D reconstruction and simulation techniques can contribute to distinguish between (static) patterns and (dynamic) rhythms of apoptosis. By deciphering novel patterns and rhythms of developmental apoptosis, our reconstructions help to reconcile seemingly inconsistent earlier findings in chick and mouse embryos, and to create rules for computer simulations.







Similar content being viewed by others
References
Adelmann, H. B. (1925). The development of the neural folds and cranial ganglia of the rat. Journal of Comparative Neurology, 39(1), 19–171.
Ansari, B., Coates, P. J., Greenstein, B. D., & Hall, P. A. (1993). In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. Journal of Pathology, 170(1), 1–8.
Asher, R. J., Bennett, N., & Lehmann, T. (2009). The new framework for understanding placental mammal evolution. BioEssays, 31(8), 853–864.
Barrow, J. R., Stadler, H. S., & Capecchi, M. R. (2000). Roles of Hoxa1 and Hoxa2 in patterning the early hindbrain of the mouse. Development, 127(5), 933–944.
Boissonnat, J. D. (1988). Shape reconstruction from planar cross sections. Computer Vision Graphics and Image Processing, 44, 1–29.
Bronner-Fraser, M. (1986). Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Developmental Biology, 115(1), 44–55.
Brunnett, G., Vančo, M., Haller, C., Washausen, S., Kuhn, H.-J., & Knabe, W. (2003). Visualization of cross sectional data for morphogenetic studies. In K. R. Dittrich, W. König, A. Oberweis, K. Rannenberg, & W. Wahlster (Eds.), Informatik 2003: Innovative Informatikanwendungen (Vol. 1, pp. 354–359). GI-Edition: Lecture notes in informatics, P-34. Bonn: Köllen.
Chizhikov, V. V., & Millen, K. J. (2004). Mechanisms of roof plate formation in the vertebrate CNS. Nature Reviews Neuroscience, 5(10), 808–812.
Choi, D.-S., Ward, S. J., Messaddeq, N., Launay, J.-M., & Maroteaux, L. (1997). 5-HT2B receptor-mediated serotonin morphogenetic functions in mouse cranial neural crest and myocardiac cells. Development, 124(9), 1745–1755.
Clarke, J. (2009). Live imaging of development in fish embryos. Seminars in Cell & Developmental Biology, 20(8), 942–946.
Copp, A. J., Stanier, P., & Greene, N. D. E. (2013). Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurology, 12(8), 799–810.
de Medeiros, G., Balázs, B., & Hufnagel, L. (2016). Light-sheet imaging of mammalian development. Seminars in Cell & Developmental Biology, 55, 148–155.
Diamantis, A., Magiorkinis, E., Sakorafas, G. H., & Androutsos, G. (2008). A brief history of apoptosis: From ancient to modern times. Onkologie, 31(12), 702–706.
Dunty, W. C., Jr., Zucker, R. M., & Sulik, K. K. (2002). Hindbrain and cranial nerve dysmorphogenesis result from acute maternal ethanol administration. Developmental Neuroscience, 24(4), 328–342.
Duprez, L., Wirawan, E., Vanden Berghe, T., & Vandenabeele, P. (2009). Major cell death pathways at a glance. Microbes and Infection, 11(13), 1050–1062.
Ellies, D. L., Church, V., Francis-West, P., & Lumsden, A. (2000). The WNT antagonist cSFRP2 modulates programmed cell death in the developing hindbrain. Development, 127(24), 5285–5295.
Erickson, C. A., & Weston, J. A. (1983). An SEM analysis of neural crest migration in the mouse. Journal of Embryology and Experimental Morphology, 74, 97–118.
Ernst, M. (1926). Über Untergang von Zellen während der normalen Entwicklung bei Wirbeltieren. Zeitschrift für Anatomie und Entwicklungsgeschichte, 79(1–2), 228–262.
Farlie, P. G., Kerr, R., Thomas, P., Symes, T., Minichiello, J., Hearn, C. J., et al. (1999). A paraxial exclusion zone creates patterned cranial neural crest cell outgrowth adjacent to rhombomeres 3 and 5. Developmental Biology, 213(1), 70–84.
Gammill, L. S., & Bronner-Fraser, M. (2003). Neural crest specification: migrating into genomics. Nature Reviews Neuroscience, 4(10), 795–805.
Gavrieli, Y., Sherman, Y., & Ben-Sasson, S. A. (1992). Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. Journal of Cell Biology, 119(3), 493–501.
Geelen, J. A. G., & Langman, J. (1977). Closure of the neural tube in the cephalic region of the mouse embryo. Anatomical Record, 189(4), 625–640.
Geelen, J. A. G., & Langman, J. (1979). Ultrastructural observations on closure of the neural tube in the mouse. Anatomy and Embryology, 156(1), 73–88.
Glücksmann, A. (1930). Über die Bedeutung von Zellvorgängen für die Formbildung epithelialer Organe. Zeitschrift für Anatomie und Entwicklungsgeschichte, 93(1–2), 35–92.
Glücksmann, A. (1940). Development and differentiation of the tadpole eye. British Journal of Ophthalmology, 24(4), 153–178.
Glücksmann, A. (1951). Cell deaths in normal vertebrate ontogeny. Biological Reviews of the Cambridge Philosophical Society, 26(1), 59–86.
Graham, A., Francis-West, P., Brickell, P., & Lumsden, A. (1994). The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature, 372(6507), 684–686.
Graham, A., Heyman, I., & Lumsden, A. (1993). Even-numbered rhombomeres control the apoptotic elimination of neural crest cells from odd-numbered rhombomeres in the chick hindbrain. Development, 119(1), 233–245.
Grasl-Kraupp, B., Ruttkay-Nedecky, B., Koudelka, H., Bukowska, K., Bursch, W., & Schulte-Hermann, R. (1995). In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology, 21(5), 1465–1468.
Greene, N. D. E., Stanier, P., & Copp, A. J. (2009). Genetics of human neural tube defects. Human Molecular Genetics, 18(2), R113–R129.
Häcker, G. (2000). The morphology of apoptosis. Cell and Tissue Research, 301(1), 5–17.
Holmdahl, D. E. (1928). Die Entstehung und weitere Entwicklung der Neuralleiste (Ganglienleiste) bei Vögeln und Säugetieren. Zeitschrift für mikroskopisch-anatomische Forschung, 14, 99–298.
Homma, S., Yaginuma, H., & Oppenheim, R. W. (1994). Programmed cell death during the earliest stages of spinal cord development in the chick embryo: A possible means of early phenotypic selection. Journal of Comparative Neurology, 345(3), 377–395.
Houston, M. L. (1968). The early brain development of the dog. Journal of Comparative Neurology, 134(3), 371–383.
Hoving, E. W., Vermeij-Keers, C., Mommaas-Kienhuis, A. M., & Hartwig, N. G. (1990). Separation of neural and surface ectoderm after closure of the rostral neuropore. Anatomy and Embryology, 182(5), 455–463.
Hu, Z., Yuri, K., Ozawa, H., Lu, H., & Kawata, M. (1997). The in vivo time course for elimination of adrenalectomy-induced apoptotic profiles from the granule cell layer of the rat hippocampus. Journal of Neuroscience, 17(11), 3981–3989.
Hutson, M. S., Leung, M. C. K., Baker, N. C., Spencer, R. M., & Knudsen, T. B. (2017). Computational model of secondary palate fusion and disruption. Chemical Research in Toxicology, 30(4), 965–979.
Isner, J. M., Kearney, M., Bortman, S., & Passeri, J. (1995). Apoptosis in human atherosclerosis and restenosis. Circulation, 91(11), 2703–2711.
Jeffs, P., Jaques, K., & Osmond, M. (1992). Cell death in cranial neural crest development. Anatomy and Embryology, 185(6), 583–588.
Johnson, C. Y., Honein, M. A., Flanders, W. D., Howards, P. P., Oakley, G. P., & Rasmussen, S. A. (2012). Pregnancy termination following prenatal diagnosis of anencephaly or spina bifida: A systematic review of the literature. Birth Defects Research Part A: Clinical and Molecular Teratology, 94(11), 857–863.
Kaufman, M. H., Brune, R. M., Baldock, R. A., Bard, J. B. L., & Davidson, D. (1997). Computer-aided 3-D reconstruction of serially sectioned mouse embryos: its use in integrating anatomical organization. International Journal of Developmental Biology, 41(2), 223–233.
Kerr, J. F. R. (2002). History of the events leading to the formulation of the apoptosis concept. Toxicology, 181–182, 471–474.
Kerr, J. F. R., Wyllie, A. H., & Currie, A. R. (1972). Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer, 26(4), 239–257.
Kienel, E., Vančo, M., Brunnett, G., Kowalski, T., Clauß, R., & Knabe, W. (2008). A framework for the visualization of cross sectional data in biomedical research. In L. Linsen, H. Hagen, & B. Hamann (Eds.), Visualization in medicine and life sciences. Mathematics and visualization (pp. 77–97). Berlin: Springer.
Kiraz, Y., Adan, A., Kartal Yandim, M., & Baran, Y. (2016). Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biology, 37(7), 8471–8486.
Kitanaka, C., & Kuchino, Y. (1999). Caspase-independent programmed cell death with necrotic morphology. Cell Death and Differentiation, 6(6), 508–515.
Knabe, W., & Kuhn, H.-J. (1998). Pattern of cell death during optic cup formation in the tree shrew Tupaia belangeri. Journal of Comparative Neurology, 401(3), 352–366.
Knabe, W., Süss, M., & Kuhn, H.-J. (2000). The patterns of cell death and of macrophages in the developing forebrain of the tree shrew Tupaia belangeri. Anatomy and Embryology, 201(3), 157–168.
Knabe, W., & Washausen, S. (2015). Early development of the nervous system of the eutherian Tupaia belangeri. Primate Biology. https://doi.org/10.5194/pb-2-25-2015.
Knabe, W., Washausen, S., Brunnett, G., & Kuhn, H.-J. (2002). Use of “reference series” to realign histological serial sections for three-dimensional reconstructions of the positions of cellular events in the developing brain. Journal of Neuroscience Methods, 121, 169–180.
Knabe, W., Washausen, S., Brunnett, G., & Kuhn, H.-J. (2004). Rhombomere-specific patterns of apoptosis in the tree shrew Tupaia belangeri. Cell and Tissue Research, 316(1), 1–13.
Knabe, W., Washausen, S., Happel, N., & Kuhn, H.-J. (2007). Development of starburst cholinergic amacrine cells in the retina of Tupaia belangeri. Journal of Comparative Neurology, 502(4), 584–597.
Knabe, W., Washausen, S., Happel, N., & Kuhn, H.-J. (2008). Diversity in mammalian chiasmatic architecture: Ipsilateral axons are deflected at glial arches in the prechiasmatic optic nerve of the eutherian Tupaia belangeri. Journal of Comparative Neurology, 508(3), 437–457.
Kocsis, E., Trus, B. L., Steer, C. J., Bisher, M. E., & Steven, A. C. (1991). Image averaging of flexible fibrous macromolecules: The clathrin triskelion has an elastic proximal segment. Journal of Structural Biology, 107(1), 6–14.
Kotch, L. E., & Sulik, K. K. (1992). Patterns of ethanol-induced cell death in the developing nervous system of mice; neural fold states through the time of anterior neural tube closure. International Journal of Developmental Neuroscience, 10(4), 273–279.
Krzic, U., Gunther, S., Saunders, T. E., Streichan, S. J., & Hufnagel, L. (2012). Multiview light-sheet microscope for rapid in toto imaging. Nature Methods, 9(7), 730–733.
Labat-Moleur, F., Guillermet, C., Lorimier, P., Robert, C., Lantuejoul, S., Brambilla, E., et al. (1998). TUNEL apoptotic cell detection in tissue sections: Critical evaluation and improvement. Journal of Histochemistry and Cytochemistry, 46(3), 327–334.
Lawson, A., & England, M. A. (1998). Neural fold fusion in the cranial region of the chick embryo. Developmental Dynamics, 212(4), 473–481.
Lawson, A., Schoenwolf, G. C., England, M. A., Addai, F. K., & Ahima, R. S. (1999). Programmed cell death and the morphogenesis of the hindbrain roof plate in the chick embryo. Anatomy and Embryology, 200(5), 509–519.
Luengo-Oroz, M. A., Ledesma-Carbayo, M. J., Peyriéras, N., & Santos, A. (2011). Image analysis for understanding embryo development: A bridge from microscopy to biological insights. Current Opinion in Genetics & Development, 21(5), 630–637.
Lumsden, A., Sprawson, N., & Graham, A. (1991). Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development, 113(4), 1281–1291.
Maden, M., Graham, A., Gale, E., Rollinson, C., & Zile, M. (1997). Positional apoptosis during vertebrate CNS development in the absence of endogenous retinoids. Development, 124(14), 2799–2805.
Marin-Padilla, M. (1970). The closure of the neural tube in the golden hamster. Teratology, 3(1), 39–45.
Marin-Riera, M., Brun-Usan, M., Zimm, R., Välikangas, T., & Salazar-Ciudad, I. (2016). Computational modeling of development by epithelia, mesenchyme and their interactions: A unified model. Bioinformatics, 32(2), 219–225.
Massa, V., Savery, D., Ybot-Gonzalez, P., Ferraro, E., Rongvaux, A., Cecconi, F., et al. (2009). Apoptosis is not required for mammalian neural tube closure. Proceedings of the National Academy of Sciences of the United States of America, 106(20), 8233–8238.
Morriss-Kay, G. M. (1981). Growth and development of pattern in the cranial neural epithelium of rat embryos during neurulation. Journal of Embryology and Experimental Morphology, 65(Suppl), 225–241.
Müller, F., & O’Rahilly, R. (1985). The first appearance of the neural tube and optic primordium in the human embryo at stage 10. Anatomy and Embryology, 172(2), 157–169.
Nichols, D. H. (1981). Neural crest formation in the head of the mouse embryo as observed using a new histological technique. Journal of Embryology and Experimental Morphology, 64, 105–120.
Nichols, D. H. (1986). Formation and distribution of neural crest mesenchyme to the first pharyngeal arch region of the mouse embryo. The American Journal of Anatomy, 176(2), 221–231.
Nicotera, P., & Leist, M. (1997). Energy supply and the shape of death in neurons and lymphoid cells. Cell Death and Differentiation, 4(6), 435–442.
Niederreither, K., Vermot, J., Schuhbaur, B., Chambon, P., & Dollé, P. (2000). Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development, 127(1), 75–85.
Nikolopoulou, E., Galea, G. L., Rolo, A., Greene, N. D. E., & Copp, A. J. (2017). Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development, 144(4), 552–566.
Nissen, S. B., Perera, M., Gonzalez, J. M., Morgani, S. M., Jensen, M. H., Sneppen, K., et al. (2017). Four simple rules that are sufficient to generate the mammalian blastocyst. PLoS Biology, 15(7), e2000737.
Nonomura, K., Yamaguchi, Y., Hamachi, M., Koike, M., Uchiyama, Y., Nakazato, K., et al. (2013). Local apoptosis modulates early mammalian brain development through the elimination of morphogen-producing cells. Developmental Cell, 27(6), 621–634.
Ostrom, L., Tang, M.-J., Gruss, P., & Dressler, G. R. (2000). Reduced Pax2 gene dosage increases apoptosis and slows the progression of renal cystic disease. Developmental Biology, 219(2), 250–258.
Penaloza, C., Lin, L., Lockshin, R. A., & Zakeri, Z. (2006). Cell death in development: shaping the embryo. Histochemistry and Cell Biology, 126(2), 149–158.
Power, R. M., & Huisken, J. (2017). A guide to light-sheet fluorescence microscopy for multiscale imaging. Nature Methods, 14(4), 360–373.
Rabinovitch, M. (1995). Professional and non-professional phagocytes: an introduction. Trends in Cell Biology, 5(3), 85–87.
Rasband, W. S. (1997–2016). ImageJ. US National Institutes of Health, Bethesda, Maryland, USA. https://imagej.nih.gov/ij/.
Romeis, B. (1989). Heidenhains Eisenhämatoxylin. In P. Böck (Ed.), Mikroskopische Technik (pp. 219–220). Baltimore: Urban-Schwarzenberg.
Römer, S., Bender, H., Knabe, W., Zimmermann, E., Rübsamen, R., Seeger, J., et al. (2018). Neural progenitors in the developing neocortex of the northern tree shrew (Tupaia belangeri) show a closer relationship to gyrencephalic primates than to lissencephalic rodents. Frontiers in Neuroanatomy, 12, 29.
Rossel, M., & Capecchi, M. R. (1999). Mice mutant for both Hoxa1 and Hoxb1 show extensive remodeling of the hindbrain and defects in craniofacial development. Development, 126(22), 5027–5040.
Ruijter, J. M., Soufan, A. T., Hagoort, J., & Moorman, A. F. (2004). Molecular imaging of the embryonic heart: Fables and facts on 3D imaging of gene expression patterns. Birth Defects Research Part C: Embryo Today, 72(3), 224–240.
Ryoo, H. D., & Steller, H. (2005). Developmental apoptosis in health and disease. In M. Holcik, E. C. LaCasse, A. E. MacKenzie, & R. G. Korneluk (Eds.), Apoptosis in health and disease: Clinical and therapeutic aspects (pp. 49–74). Cambridge: Cambridge University Press.
Sadler, T. W. (1978). Distribution of surface coat material on fusing neural folds of mouse embryos during neurulation. Anatomical Record, 191(3), 345–349.
Saiz, N., Plusa, B., & Hadjantonakis, A.-K. (2015). Single cells get together: High-resolution approaches to study the dynamics of early mouse development. Seminars in Cell & Developmental Biology, 47–48, 92–100.
Sanders, E. J., & Wride, M. A. (1995). Programmed cell death in development. International Review of Cytology, 163, 105–173.
Saunders, J. W., Jr. (1966). Death in embryonic systems. Science, 154(3749), 604–612.
Schlüter, G. (1973). Ultrastructural observations on cell necrosis during formation of the neural tube in mouse embryos. Zeitschrift für Anatomie und Entwicklungsgeschichte, 141(3), 251–264.
Schoenwolf, G. C. (1979). Observations on closure of the neuropores in the chick embryo. The American Journal of Anatomy, 155(4), 445–465.
Selçuki, M., Vatansever, S., Umur, A. S., Temiz, C., & Sayin, M. (2008). Apoptosis seems to be the major process while surface and neural ectodermal layers detach during neurulation. Childs Nervous System, 24(5), 577–580.
Serbedzija, G. N., Dickinson, M., & McMahon, A. P. (1996). Cell death in the CNS of the Wnt-1 mutant mouse. Journal of Neurobiology, 31(3), 275–282.
Smith, A., & Graham, A. (2001). Restricting Bmp-4 mediated apoptosis in hindbrain neural crest. Developmental Dynamics, 220(3), 276–283.
Sulik, K. K., Cook, C. S., & Webster, W. S. (1988). Teratogens and craniofacial malformations: relationships to cell death. Development, 103(Suppl), 213–231.
Süss, M., Washausen, S., Kuhn, H.-J., & Knabe, W. (2002). High resolution scanning and three-dimensional reconstruction of cellular events in large objects during brain development. Journal of Neuroscience Methods, 113(2), 147–158.
Tait, S. W., & Green, D. R. (2008). Caspase-independent cell death: leaving the set without the final cut. Oncogene, 27(50), 6452–6461.
Takahashi, K., Nuckolls, G. H., Tanaka, O., Semba, I., Takahashi, I., Dashner, R., et al. (1998). Adenovirus-mediated ectopic expression of Msx2 in even-numbered rhombomeres induces apoptotic elimination of cranial neural crest cells in ovo. Development, 125(9), 1627–1635.
Tan, S. S., & Morriss-Kay, G. (1985). The development and distribution of the cranial neural crest in the rat embryo. Cell and Tissue Research, 240(2), 403–416.
Trainor, P. A., Sobieszczuk, D., Wilkinson, D., & Krumlauf, R. (2002). Signalling between the hindbrain and paraxial tissues dictates neural crest migration pathways. Development, 129(2), 433–442.
van Straaten, H. W. M., Jaskoll, T., Rousseau, A. M. J., Terwindt-Rouwenhorst, E. A. W., Greenberg, G., Shankar, K., et al. (1993). Raphe of the posterior neural tube in the chick embryo: its closure and reopening as studied in living embryos with a high definition light microscope. Developmental Dynamics, 198(1), 65–76.
Vogt, K. C. (1842). Untersuchungen über die Entwicklungsgeschichte der Geburtshelferkröte (Alytes obstetricans). Solothurn: Jent & Gassmann.
Washausen, S., & Knabe, W. (2017). Pax2/Pax8-defined subdomains and the occurrence of apoptosis in the posterior placodal area of mice. Brain Structure and Function, 222(6), 2671–2695.
Weber, M., & Huisken, J. (2015). In vivo imaging of cardiac development and function in zebrafish using light sheet microscopy. Swiss Medical Weekly, 145, w14227.
Weil, M., Jacobson, M. D., & Raff, M. C. (1997). Is programmed cell death required for neural tube closure? Current Biology, 7(4), 281–284.
Wellmann, J. (2010). Die Form des Werdens: Eine Kulturgeschichte der Embryologie 1760–1830. Göttingen: Wallstein.
Whitehead, A. N. (1919). An enquiry concerning the principles of natural knowledge. London: Cambridge University Press.
Wu, G. F., & Perlman, S. (1999). Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. Journal of Virology, 73(10), 8771–8780.
Xu, Q., Mellitzer, G., Robinson, V., & Wilkinson, D. G. (1999). In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature, 399, 267–271.
Yamaguchi, Y., Shinotsuka, N., Nonomura, K., Takemoto, K., Kuida, K., Yosida, H., et al. (2011). Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. Journal of Cell Biology, 195(6), 1047–1060.
Acknowledgments
The monoclonal anti-Pax3 antibody developed by Charles P. Ordahl was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Washausen, S., Scheffel, T., Brunnett, G. et al. Possibilities and limitations of three-dimensional reconstruction and simulation techniques to identify patterns, rhythms and functions of apoptosis in the early developing neural tube. HPLS 40, 55 (2018). https://doi.org/10.1007/s40656-018-0222-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40656-018-0222-1