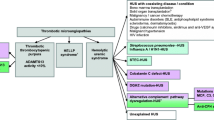
Abstract
Background
Atypical hemolytic uremic syndrome (aHUS) is characterized by platelet consumption, hemolysis, and renal injury. Eculizumab, a humanized antibody that blocks complement activity, has been successfully used in aHUS, but the best treatment schedule has not yet been clearly defined.
Methods
Herein we report our experience with eculizumab maintenance treatment, in which the interval between subsequent doses was adjusted based on classical complement pathway (CCP) activity, targeted to < 30% for the prevention of relapses. Trough circulating levels of free eculizumab were determined by an immunoenzymatic method. Genetic and serologic characteristics of the patients were also assessed.
Results
We report on 38 patients with aHUS with a median age of 25.0 years (range 0.5–60.0 years) treated with eculizumab. Once stable disease remission was obtained, the interval between eculizumab doses was extended based on target CCP activity. With this approach, presently, 22 patients regularly receive eculizumab infusion every 28 days and 16 receive it every 21. During a median observation period of 32.3 months (range 4.0–92.4 months) and a cumulative period of 1295 months, no patient relapsed. An inverse correlation between CCP activity and eculizumab circulating levels was present (r = − 0.690, p = 0.0001), with CCP activity being inhibited as long as free eculizumab was measurable in serum.
Conclusions
In patients with aHUS on eculizumab maintenance treatment, complement activity measurement can be used as a proxy for circulating levels of the drug. Monitoring complement activity allows for safe tailoring of the frequency of eculizumab administration, thus avoiding excessive drug exposure while keeping the disease in remission.
Graphical Abstract





Similar content being viewed by others
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Noris M, Remuzzi G (2009) Atypical hemolytic-uremic syndrome. N Engl J Med 361:1676–87. https://doi.org/10.1056/NEJMra0902814
Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Frémeaux-Bacchi V, Kavanagh D, Nester CM, Noris M, Pickering MC, Rodríguez de Córdoba S, Roumenina LT, Sethi S, Smith RJ (2017) Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 91:539–551. https://doi.org/10.1016/j.kint.2016.10.005
Dragon-Durey MA, Loirat C, Cloarec S, Macher MA, Blouin J, Nivet H, Weiss L, Fridman WH, Frémeaux-Bacchi V (2005) Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol 16:555–563. https://doi.org/10.1681/ASN.2004050380
Cugno M, Berra S, Depetri F, Tedeschi S, Griffini S, Grovetti E, Caccia S, Cresseri D, Messa P, Testa S, Giglio F, Peyvandi F, Ardissino G (2021) IgM autoantibodies to complement factor H in atypical hemolytic uremic syndrome. J Am Soc Nephrol. https://doi.org/10.1681/ASN.2020081224 (Online ahead of print)
Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C (2013) Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368:2169–2181. https://doi.org/10.1056/NEJMoa1208981
Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, Lieberman KV, Maringhini S, Pape L, Rees L, van de Kar NC, Vande Walle J, Ogawa M, Bedrosian CL, Licht C (2016) Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int 89:701–711. https://doi.org/10.1016/j.kint.2015.11.026
Cugno M, Gualtierotti R, Possenti I, Testa S, Tel F, Griffini S, Grovetti E, Tedeschi S, Salardi S, Cresseri D, Messa P, Ardissino G (2014) Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome. J Thromb Haemost 12:1440–1448. https://doi.org/10.1111/jth.12615
Cugno M, Tedeschi S, Ardissino G (2015) Tailored eculizumab regimen for patients with atypical hemolytic uremic syndrome: requirement for comprehensive complement analysis: comment. J Thromb Haemost 13:485–486. https://doi.org/10.1111/jth.12764
Ardissino G, Tel F, Sgarbanti M, Cresseri D, Giussani A, Griffini S, Grovetto E, Possenti I, Perrone M, Testa S, Paglialonga F, Messa P, Cugno M (2018) Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome: an update. Pediatr Nephrol 33:457–461. https://doi.org/10.1007/s00467-017-3813-2
Volokhina EB, van de Kar NC, Bergseth G, van der Velden TJ, Westra D, Wetzels JF, van den Heuvel LP, Mollnes TE (2016) Sensitive, reliable and easy-performed laboratory monitoring of eculizumab therapy in atypical hemolytic uremic syndrome. Clin Immunol 160:237–243. https://doi.org/10.1016/j.clim.2015.05.018
Gatault P, Brachet G, Ternant D, Degenne D, Récipon G, Barbet C, Gyan E, Gouilleux-Gruart V, Bordes C, Farrell A, Halimi JM, Watier H (2015) Therapeutic drug monitoring of eculizumab: rationale for an individualized dosing schedule. MAbs 7:1205–11. https://doi.org/10.1080/19420862.2015.1086049
Passot C, Sberro-Soussan R, Bertrand D, Caillard S, Schvartz B, Domenger C, Contin-Bordes C, Paintaud G, Halimi JM, Ternant D, Gatault P (2021) Feasibility and safety of tailored dosing schedule for eculizumab based on therapeutic drug monitoring: lessons from a prospective multicentric study. Br J Clin Pharmacol 87:2236–46. https://doi.org/10.1111/bcp.14627
Jodele S, Fukuda T, Mizuno K, Vinks AA, Laskin BL, Goebel J, Dixon BP, Chima RS, Hirsch R, Teusink A, Lazear D, Lane A, Myers KC, Dandoy CE, Davies SM (2016) Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 22:307–315. https://doi.org/10.1016/j.bbmt.2015.10.002
Ardissino G, Testa S, Possenti I, Tel F, Paglialonga F, Salardi S, Tedeschi S, Belingheri M, Cugno M (2014) Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis 64:633–637. https://doi.org/10.1053/j.ajkd.2014.01.434
Ardissino G, Possenti I, Tel F, Testa S, Salardi S, Ladisa V (2015) Discontinuation of eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am J Kidney Dis 66:172–173. https://doi.org/10.1053/j.ajkd.2015.04.010
Galbusera M, Noris M, Gastoldi S, Bresin E, Mele C, Breno M, Cuccarolo P, Alberti M, Valoti E, Piras R, Donadelli R, Vivarelli M, Murer L, Pecoraro C, Ferrari E, Perna A, Benigni A, Portalupi V, Remuzzi G (2019) An ex vivo test of complement activation on endothelium for individualized eculizumab therapy in hemolytic uremic syndrome. Am J Kidney Dis 74:56–72. https://doi.org/10.1053/j.ajkd.2018.11.012
Acknowledgements
The authors are grateful to “Progetto Alice Onlus, Associazione per la lotta alla Sindrome Emolitico Uremica” for the valuable support provided to perform this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Designed the study MC and GA. Collected clinical data MC, VC and GA. Performed biological analyses SG, EG, GP, and LP. Designed and performed statistical analyses MC, EC and GA. Drafted the initial version of the manuscript MC and GA. Revised the manuscript critically for intellectual content MC, VC, SG, EG, GP, LP, EC and GA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
G. Ardissino reports consultancy agreements with Alexion and Alnylam; scientific advisory board membership with Alexion Inc. All remaining authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The study was approved by the local review board and was conducted according to the ethical principles contained in the 2013 revision of the Declaration of Helsinki and the code of Good Clinical Practice.
Consent to participate
Written informed consent was obtained from all individual adult participants and from the parents of pediatric patients included in the study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cugno, M., Capone, V., Griffini, S. et al. Eculizumab treatment in atypical hemolytic uremic syndrome: correlation between functional complement tests and drug levels. J Nephrol 35, 1205–1211 (2022). https://doi.org/10.1007/s40620-021-01187-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-021-01187-8