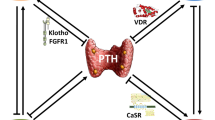
Abstract
Increasing survival in the chronic kidney disease (CKD) population exposes the bone to the cumulative detrimental sequelae of CKD, now defined physiologically and histopathologically as chronic kidney disease mineral bone disorder (CKD-BMD). This disorder is increasingly recognized as a “nontraditional” driver of morbidity and mortality and presents an opportunity to improve CKD outcomes via research. However, recent advances in the literature on this topic have not yet been collected into a single review. Therefore, this report aims to discuss the disordered renal-bone axis in CKD-BMD, molecular and hormonal drivers, novel treatment strategies, and forthcoming research in a clinician-directed format. A key novel topic will be the unique impact of uric acid on CKD-BMD, which is poised to apply extensive existing research in the uric acid domain to benefit the CKD-BMD population.

Similar content being viewed by others
References
Covic A, Kanbay M, Voroneanu L, Turgut F, Serban DN, Serban IL et al (2010) Vascular calcification in chronic kidney disease. Clin Sci (Lond). 119(3):111–121
Sag AA, Covic A, London G, Vervloet M, Goldsmith D, Gorriz JL et al (2016) Clinical imaging of vascular disease in chronic kidney disease. Int Urol Nephrol 48(6):827–837
Kanbay M, Goldsmith D, Akcay A, Covic A (2009) Phosphate—the silent stealthy cardiorenal culprit in all stages of chronic kidney disease: a systematic review. Blood Purif 27(2):220–230
Afsar B, Turkmen K, Covic A, Kanbay M (2014) An update on coronary artery disease and chronic kidney disease. Int J Nephrol 2014:767424
Kanbay M, Nicoleta M, Selcoki Y, Ikizek M, Aydin M, Eryonucu B et al (2010) Fibroblast growth factor 23 and fetuin A are independent predictors for the coronary artery disease extent in mild chronic kidney disease. Clin J Am Soc Nephrol 5(10):1780–1786
Kanbay M, Vervloet M, Cozzolino M, Siriopol D, Covic A, Goldsmith D et al (2017) Novel Faces of Fibroblast Growth Factor 23 (FGF23): iron Deficiency, Inflammation, Insulin Resistance, Left Ventricular Hypertrophy, Proteinuria and Acute Kidney Injury. Calcif Tissue Int 100(3):217–228
Kanbay M, Solak Y, Siriopol D, Aslan G, Afsar B, Yazici D et al (2016) Sclerostin, cardiovascular disease and mortality: a systematic review and meta-analysis. Int Urol Nephrol 48(12):2029–2042
Kanbay M, Siriopol D, Saglam M, Kurt YG, Gok M, Cetinkaya H et al (2014) Serum sclerostin and adverse outcomes in nondialyzed chronic kidney disease patients. J Clin Endocrinol Metab 99(10):E1854–E1861
Hruska KA, Sugatani T, Agapova O, Fang Y (2017) The chronic kidney disease—mineral bone disorder (CKD-MBD): advances in pathophysiology. Bone 100:80–86
Hahn K, Kanbay M, Lanaspa MA, Johnson RJ, Ejaz AA (2017) Serum uric acid and acute kidney injury: a mini review. J Adv Res. 8(5):529–536
Jensen T, Niwa K, Hisatome I, Kanbay M, Andres-Hernando A, Roncal-Jimenez CA et al (2018) Increased serum uric acid over five years is a risk factor for developing fatty liver. Sci Rep. 8(1):11735
Kanbay M, Jensen T, Solak Y, Le M, Roncal-Jimenez C, Rivard C et al (2016) Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur J Intern Med. 29:3–8
Takir M, Kostek O, Ozkok A, Elcioglu OC, Bakan A, Erek A et al (2015) Lowering uric acid with allopurinol improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia. J Investig Med 63(8):924–929
Kanbay M, Yilmaz MI, Sonmez A, Turgut F, Saglam M, Cakir E et al (2011) Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. Am J Nephrol 33(4):298–304
Kanbay M, Siriopol D, Nistor I, Elcioglu OC, Telci O, Takir M et al (2014) Effects of allopurinol on endothelial dysfunction: a meta-analysis. Am J Nephrol 39(4):348–356
Kanbay M, Solak Y, Dogan E, Lanaspa MA, Covic A (2010) Uric acid in hypertension and renal disease: the chicken or the egg? Blood Purif 30(4):288–295
Toyama T, Furuichi K, Shimizu M, Hara A, Iwata Y, Sakai N et al (2015) Relationship between serum uric acid levels and chronic kidney disease in a japanese cohort with normal or mildly reduced kidney function. PLoS One 10(9):e0137449
Kanbay M, Yilmaz MI, Sonmez A, Solak Y, Saglam M, Cakir E et al (2012) Serum uric acid independently predicts cardiovascular events in advanced nephropathy. Am J Nephrol 36(4):324–331
Kanbay M, Ikizek M, Solak Y, Selcoki Y, Uysal S, Armutcu F et al (2011) Uric acid and pentraxin-3 levels are independently associated with coronary artery disease risk in patients with stage 2 and 3 kidney disease. Am J Nephrol 33(4):325–331
von Lueder TG, Girerd N, Atar D, Agewall S, Lamiral Z, Kanbay M et al (2015) Serum uric acid is associated with mortality and heart failure hospitalizations in patients with complicated myocardial infarction: findings from the high-risk myocardial infarction database initiative. Eur J Heart Fail 17(11):1144–1151
Turak O, Ozcan F, Tok D, Isleyen A, Sokmen E, Tasoglu I et al (2013) Serum uric acid, inflammation, and nondipping circadian pattern in essential hypertension. J Clin Hypertens (Greenwich). 15(1):7–13
Cagli K, Turak O, Canpolat U, Ozcan F, Tok D, Mendi MA et al (2015) Association of serum uric acid level with blood pressure variability in newly diagnosed essential hypertension. J Clin Hypertens (Greenwich). 17(12):929–935
Kuwabara M, Kanbay M, Hisatome I (2018) Uric acid and hypertension because of arterial stiffness. Hypertension 72(3):582–584
Lundquist AL, Nigwekar SU (2016) Optimal management of bone mineral disorders in chronic kidney disease and end stage renal disease. Curr Opin Nephrol Hypertens 25(2):120–126
Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA et al (2007) Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71(1):31–38
Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H et al (2011) Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79(12):1370–1378
Farrow EG, Davis SI, Summers LJ, White KE (2009) Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J Am Soc Nephrol 20(5):955–960
Juppner H, Wolf M, Salusky IB (2010) FGF-23: more than a regulator of renal phosphate handling? J Bone Miner Res 25(10):2091–2097
Vervloet MG, Sezer S, Massy ZA, Johansson L, Cozzolino M, Fouque D et al (2017) The role of phosphate in kidney disease. Nat Rev Nephrol. 13(1):27–38
Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K et al (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: improving Global Outcomes (KDIGO). Kidney Int 69(11):1945–1953
Goodman WG (2005) The evolution of assays for parathyroid hormone. Semin Dial 18(4):296–301
Ferreira A, Drueke TB (2000) Biological markers in the diagnosis of the different forms of renal osteodystrophy. Am J Med Sci 320(2):85–89
Reiss AB, Miyawaki N, Moon J, Kasselman LJ, Voloshyna I, D’Avino R Jr et al (2018) CKD, arterial calcification, atherosclerosis and bone health: inter-relationships and controversies. Atherosclerosis. 278:49–59
Kanbay M, Afsar B, Gusbeth-Tatomir P, Covic A (2010) Arterial stiffness in dialysis patients: where are we now? Int Urol Nephrol 42(3):741–752
Bikle DD (2014) Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 21(3):319–329
Peng H, Li H, Li C, Chao X, Zhang Q, Zhang Y (2013) Association between vitamin D insufficiency and elevated serum uric acid among middle-aged and elderly Chinese Han women. PLoS One 8(4):e61159
Yilmaz H, Kaya M, Sahin M, Delibasi T (2012) Is vitamin D status a predictor glycaemic regulation and cardiac complication in type 2 diabetes mellitus patients? Diabetes Metab Syndr. 6(1):28–31
Vanholder R, Patel S, Hsu CH (1993) Effect of uric acid on plasma levels of 1,25(OH)2D in renal failure. J Am Soc Nephrol 4(4):1035–1038
Takahashi S, Yamamoto T, Moriwaki Y, Tsutsumi Z, Yamakita J, Higashino K (1998) Decreased serum concentrations of 1,25(OH)2-vitamin D3 in patients with gout. Metabolism. 47(3):336–338
Hsu CH, Patel SR, Young EW, Vanholder R (1991) Effects of purine derivatives on calcitriol metabolism in rats. Am J Physiol 260(4 Pt 2):F596–F601
Chen W, Roncal-Jimenez C, Lanaspa M, Gerard S, Chonchol M, Johnson RJ et al (2014) Uric acid suppresses 1 alpha hydroxylase in vitro and in vivo. Metabolism. 63(1):150–160
Thakkinstian A, Anothaisintawee T, Chailurkit L, Ratanachaiwong W, Yamwong S, Sritara P et al (2015) Potential causal associations between vitamin D and uric acid: bidirectional mediation analysis. Sci Rep. 5:14528
Chin KY, Nirwana SI, Ngah WZ (2015) Significant association between parathyroid hormone and uric acid level in men. Clin Interv Aging 10:1377–1380
Hui JY, Choi JW, Mount DB, Zhu Y, Zhang Y, Choi HK (2012) The independent association between parathyroid hormone levels and hyperuricemia: a national population study. Arthritis Res Ther. 14(2):R56
Alemzadeh R, Kichler J (2016) Uric acid-induced inflammation is mediated by the parathyroid hormone: 25-hydroxyvitamin D ratio in obese adolescents. Metab Syndr Relat Disord. 14(3):167–174
Saleh F, Jorde R, Sundsfjord J, Haug E, Figenschau Y (2006) Causes of secondary hyperparathyroidism in a healthy population: the Tromso study. J Bone Miner Metab 24(1):58–64
Hisatome I, Ishimura M, Sasaki N, Yamakawa M, Kosaka H, Tanaka Y et al (1992) Renal handling of urate in two patients with hyperuricemia and primary hyperparathyroidism. Intern Med 31(6):807–811
Westerdahl J, Valdemarsson S, Lindblom P, Bergenfelz A (2001) Urate and arteriosclerosis in primary hyperparathyroidism. Clin Endocrinol (Oxf) 54(6):805–811
Bergenfelz A, Bladstrom A, Their M, Nordenstrom E, Valdemarsson S, Westerdahl J (2007) Serum levels of uric acid and diabetes mellitus influence survival after surgery for primary hyperparathyroidism: a prospective cohort study. World J Surg. 31(7):1393–1400 (discussion 401-2)
Valdemarsson S, Lindblom P, Bergenfelz A (1998) Metabolic abnormalities related to cardiovascular risk in primary hyperparathyroidism: effects of surgical treatment. J Intern Med 244(3):241–249
Christensson T (1977) Serum urate in subjects with hypercalcaemic hyperparathyroidism. Clin Chim Acta 80(3):529–533
Dalbeth N, Horne A, Gamble GD, Ames R, Mason B, McQueen FM et al (2009) The effect of calcium supplementation on serum urate: analysis of a randomized controlled trial. Rheumatology (Oxford) 48(2):195–197
Miller PD, Schwartz EN, Chen P, Misurski DA, Krege JH (2007) Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int 18(1):59–68
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y et al (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19(3):429–435
Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB (2003) Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64(6):2272–2279
Mirza MA, Larsson A, Lind L, Larsson TE (2009) Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis. 205(2):385–390
Sakoh T, Nakayama M, Tsuchihashi T, Yoshitomi R, Tanaka S, Katafuchi E et al (2016) Associations of fibroblast growth factor 23 with urate metabolism in patients with chronic kidney disease. Metabolism. 65(10):1498–1507
Asicioglu E, Kahveci A, Arikan H, Koc M, Tuglular S, Ozener C (2014) Fibroblast growth factor-23 levels are associated with uric acid but not carotid intima media thickness in renal transplant recipients. Transplant Proc. 46(1):180–183
Gutierrez OM, Wolf M, Taylor EN (2011) Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the health professionals follow-up study. Clin J Am Soc Nephrol 6(12):2871–2878
Wolf M (2010) Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol 21(9):1427–1435
Andres M, Quintanilla MA, Sivera F, Sanchez-Paya J, Pascual E, Vela P et al (2016) Silent monosodium urate crystal deposits are associated with severe coronary calcification in asymptomatic hyperuricemia: an exploratory study. Arthritis Rheumatol. 68(6):1531–1539
Kim H, Kim SH, Choi AR, Kim S, Choi HY, Kim HJ et al (2017) Asymptomatic hyperuricemia is independently associated with coronary artery calcification in the absence of overt coronary artery disease: a single-center cross-sectional study. Medicine (Baltimore). 96(14):e6565
Mehta T, Nuccio E, McFann K, Madero M, Sarnak MJ, Jalal D (2015) Association of uric acid with vascular stiffness in the framingham heart study. Am J Hypertens 28(7):877–883
Lee CT, Chua S, Hsu CY, Tsai YC, Ng HY, Kuo CC et al (2013) Biomarkers associated with vascular and valvular calcification in chronic hemodialysis patients. Dis Markers 34(4):229–235
Andrews ES, Perrenoud L, Nowak KL, You Z, Pasch A, Chonchol M et al (2018) Examining the effects of uric acid-lowering on markers vascular of calcification and CKD-MBD; A post hoc analysis of a randomized clinical trial. PLoS One 13(10):e0205831
Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N et al (2007) Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol 39(4):1227–1233
Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincon A et al (2010) Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 5(8):1388–1393
Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I et al (2011) A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol 6(8):1887–1894
Nabipour I, Sambrook PN, Blyth FM, Janu MR, Waite LM, Naganathan V et al (2011) Serum uric acid is associated with bone health in older men: a cross-sectional population-based study. J Bone Miner Res 26(5):955–964
Ahn SH, Lee SH, Kim BJ, Lim KH, Bae SJ, Kim EH et al (2013) Higher serum uric acid is associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in healthy postmenopausal women. Osteoporos Int 24(12):2961–2970
Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y (2009) Oxidative stress in bone remodelling and disease. Trends Mol Med. 15(10):468–477
Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS et al (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106(3):852–859
Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P et al (2003) Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab 88(4):1523–1527
Makovey J, Macara M, Chen JS, Hayward CS, March L, Seibel MJ et al (2013) Serum uric acid plays a protective role for bone loss in peri- and postmenopausal women: a longitudinal study. Bone 52(1):400–406
Kim BJ, Baek S, Ahn SH, Kim SH, Jo MW, Bae SJ et al (2014) Higher serum uric acid as a protective factor against incident osteoporotic fractures in Korean men: a longitudinal study using the National Claim Registry. Osteoporos Int 25(7):1837–1844
Zhao DD, Jiao PL, Yu JJ, Wang XJ, Zhao L, Xuan Y et al (2016) Higher serum uric acid is associated with higher bone mineral density in Chinese men with type 2 diabetes mellitus. Int J Endocrinol. 2016:2528956
Chen Z, Ding Z, Fu C, Yu C, Ma G (2014) Correlation between serum uric Acid and renal function in patients with stable coronary artery disease and type 2 diabetes. J Clin Med Res. 6(6):443–450
Kamei K, Konta T, Hirayama A, Suzuki K, Ichikawa K, Fujimoto S et al (2014) A slight increase within the normal range of serum uric acid and the decline in renal function: associations in a community-based population. Nephrol Dial Transplant 29(12):2286–2292
Malmgren L, McGuigan F, Christensson A, Akesson KE (2017) Reduced kidney function is associated with BMD, bone loss and markers of mineral homeostasis in older women: a 10-year longitudinal study. Osteoporos Int 28(12):3463–3473
Zhang D, Bobulescu IA, Maalouf NM, Adams-Huet B, Poindexter J, Park S et al (2015) Relationship between serum uric Acid and bone mineral density in the general population and in rats with experimental hyperuricemia. J Bone Miner Res 30(6):992–999
Nybo M, Jespersen B, Aarup M, Ejersted C, Hermann AP, Brixen K (2013) Determinants of bone mineral density in patients on haemodialysis or peritoneal dialysis—a cross-sectional, longitudinal study. Biochem Med (Zagreb). 23(3):342–350
Denburg MR, Tsampalieros AK, de Boer IH, Shults J, Kalkwarf HJ, Zemel BS et al (2013) Mineral metabolism and cortical volumetric bone mineral density in childhood chronic kidney disease. J Clin Endocrinol Metab 98(5):1930–1938
Park SH, Jia T, Qureshi AR, Barany P, Heimburger O, Larsson TE et al (2013) Determinants and survival implications of low bone mineral density in end-stage renal disease patients. J Nephrol. 26(3):485–494
Perez-Gomez MV, Bartsch LA, Castillo-Rodriguez E, Fernandez-Prado R, Kanbay M, Ortiz A. Potential dangers of serum urate-lowering therapy. Am J Med. 2019
Barreto FC, Costa C, Reis LMD, Custodio MR. Bone biopsy in nephrology practice. J Bras Nefrol. 2018
Cheng Q, Wu X, Du Y, Hong W, Tang W, Li H et al (2018) Levels of serum sclerostin, FGF-23, and intact parathyroid hormone in postmenopausal women treated with calcitriol. Clin Interv Aging 13:2367–2374
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Grieco GE, Cataldo D, Ceccarelli E, Nigi L, Catalano G, Brusco N et al (2018) Serum levels of miR-148a and miR-21-5p are increased in Type 1 diabetic patients and correlated with markers of bone strength and metabolism. Noncoding RNA. 4(4):37
Yao CJ, Lv Y, Zhang CJ, Jin JX, Xu LH, Jiang J et al (2018) MicroRNA-185 inhibits the growth and proliferation of osteoblasts in fracture healing by targeting PTH gene through down-regulating Wnt/beta-catenin axis: in an animal experiment. Biochem Biophys Res Commun 501(1):55–63
Shilo V, Mor-Yosef Levi I, Abel R, Mihailovic A, Wasserman G, Naveh-Many T et al (2017) Let-7 and MicroRNA-148 regulate parathyroid hormone levels in secondary hyperparathyroidism. J Am Soc Nephrol 28(8):2353–2363
Jeong S, Oh JM, Oh KH, Kim IW (2017) Differentially expressed miR-3680-5p is associated with parathyroid hormone regulation in peritoneal dialysis patients. PLoS One 12(2):e0170535
Shao Y, Ren H, Lv C, Ma X, Wu C, Wang Q (2017) Changes of serum Mir-217 and the correlation with the severity in type 2 diabetes patients with different stages of diabetic kidney disease. Endocrine 55(1):130–138
Tang ZM, Fang M, Wang JP, Cai PC, Wang P, Hu LH (2014) Clinical relevance of plasma miR-21 in new-onset systemic lupus erythematosus patients. J Clin Lab Anal 28(6):446–451
Hong Q, Yu S, Geng X, Duan L, Zheng W, Fan M et al (2015) High concentrations of uric acid inhibit endothelial cell migration via miR-663 which regulates phosphatase and tensin homolog by targeting transforming growth factor-beta1. Microcirculation. 22(4):306–314
Yu S, Hong Q, Wang Y, Hou K, Wang L, Zhang Y et al (2015) High concentrations of uric acid inhibit angiogenesis via regulation of the Kruppel-like factor 2-vascular endothelial growth factor-A axis by miR-92a. Circ J 79(11):2487–2498
Evenepoel P, D’Haese P, Brandenburg V (2015) Sclerostin and DKK1: new players in renal bone and vascular disease. Kidney Int 88(2):235–240
Fang Y, Ginsberg C, Seifert M, Agapova O, Sugatani T, Register TC et al (2014) CKD-induced wingless/integration1 inhibitors and phosphorus cause the CKD-mineral and bone disorder. J Am Soc Nephrol 25(8):1760–1773
Sugatani T, Agapova OA, Fang Y, Berman AG, Wallace JM, Malluche HH et al (2017) Ligand trap of the activin receptor type IIA inhibits osteoclast stimulation of bone remodeling in diabetic mice with chronic kidney disease. Kidney Int 91(1):86–95
Sugatani T (2018) Systemic activation of activin a signaling causes chronic kidney disease-mineral bone disorder. Int J Mol Sci. 19(9):2490
Peng LN, Chou MY, Liang CK, Lee WJ, Kojima T, Lin MH et al (2018) Association between serum activin A and metabolic syndrome in older adults: potential of activin A as a biomarker of cardiometabolic disease. Exp Gerontol 111:197–202
Acknowledgement
MK The authors gratefully acknowledge use of the services and facilities of the Koç University Research Center for Translational Medicine (KUTTAM), funded by the Presidency of Turkey, Presidency of Strategy and Budget. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Presidency of Strategy and Budget.
Funding
This study was not funded by any grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Afsar, B., Sag, A.A., Oztosun, C. et al. The role of uric acid in mineral bone disorders in chronic kidney disease. J Nephrol 32, 709–717 (2019). https://doi.org/10.1007/s40620-019-00615-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-019-00615-0