Abstract
Purpose
Surgery plays a key role in the treatment of thyroid cancer (TC) patients. Locally advanced cases, however, can require an extensive surgical approach with technical issues and a high risk of complications. In these cases, a multidisciplinary evaluation should be carried out to evaluate pros and cons. The aim of this study was to share our experience, as a multidisciplinary team, in the management of patients with locally advanced TC with a particularly extensive local disease, whose surgical approach could be challenging and part of a multimodal treatment.
Methods
We retrospectively evaluated clinical, surgical, and oncologic features of all patients with locally advanced TC who had undergone multidisciplinary surgery from January 2019 to June 2020.
Results
Six patients (two cases each of poorly differentiated, papillary, and medullary TC) were included. Four out of six were suffering from symptoms related to the advanced disease. At pre-surgical evaluation, a multidisciplinary team proposed extended surgery with radical intent via cervicotomy and sternotomy, considering other therapies not feasible or probably ineffective without it. No one passed away in intra- or perioperative time. At the end of follow-up (median 2.6 years), all patients presented a remission of symptoms due to the advanced disease, four patients were submitted to adjuvant therapies and only one patient died for a cause unrelated to the disease.
Conclusion
This series of very advanced TCs shows the effectiveness of a surgery performed by a multidisciplinary team in controlling symptoms, allowing adjuvant therapies, and improving the survival of patients whose cases would otherwise be very difficult to manage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer (TC) is the most frequent endocrine tumor, accounting for about 22 new cases per 100.000 people every year [1]. Malignant transformation can happen both in follicular and parafollicular thyroid cells. Differentiated thyroid cancer (DTC), that represents 90% of thyroid malignancies and includes papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), poorly differentiated thyroid cancer (PDTC), and anaplastic thyroid cancer (ATC), originates from follicular cells, while medullary thyroid cancer (MTC) originates from parafollicular cells [2, 3].
Although an excellent prognosis has been widely recognized for thyroid malignancies [4], metastatic DTC, as well as specific hystotypes, namely, ATC, PDTC, or MTC, have worse prognosis [4, 5]. Durante et al. reported an overall survival of 42% at 10 years in patients with metastatic DTC at diagnosis, which dropped to 10% in radiorefractory cases [6]. In PDTC, the 5 year overall survival was recently reported to range from 63.6 to 72.8% [7, 8] and only one-third of patients reached clinical remission during their clinical course [8]. A slightly better clinical outcome was recently shown by Matrone et al. [9] in patients with MTC, although almost 50% of patients did not experience an excellent response during a median follow-up of 7 years.
Surgery plays a key role in the therapeutic management of patients with TC even in locally advanced or metastatic stage [4, 10]. In these latter cases, especially when the tumor is not confined to the neck, the role of surgical resection is still being discussed [11]. Moreover, the definition of locally advanced TC, albeit intuitive, is not standardized, as well as the definition of “unresectable” cases [11]. Finally, an extensive surgical treatment could be controversial both for technical issues and the risk of complications in patients who often present with multiple comorbidities [11, 12]. In these cases, systemic therapies could be considered and represent the standard of care [13].
The aim of this study was to share our experience, as a multidisciplinary team, in the management of patients with locally advanced TC with a particularly extensive local disease whose surgical treatment could be challenging and not resolving but at the same time necessary to make other medical treatments possible.
Materials and methods
We retrospectively reviewed patients with locally advanced TC, in whom the surgical approach of the disease needed the presence of different type of surgeons (i.e. vascular, cardiovascular, endocrine, and thoracic surgeon) because of the local aggressiveness. All patients were preoperatively evaluated by a multidisciplinary team comprising of an endocrinologist, an anesthesiologist, and surgeons of these different specialties from January 2019 to June 2020. The cases included in the present study were only those in whom surgical treatment (although extensive) was considered feasible after multidisciplinary evaluation. Conversely, we excluded all the advanced cases in whom surgical treatment was not feasible according to the judgment of different surgeons.
We analyzed demographic, clinical, surgical, and pathological data of all patients using our medical records. All patients included in this study had a complete preoperative work-up. In this regard, they were evaluated with a total body CT-scan, neck ultrasound and, when necessary, gastroscopy, bronchoscopy, and cavography. As for policy of our hospital, all patients signed an informed consent for the use of their clinical and biochemical data for research purposes. They also signed an informed consent to proceed with the suggested interventional treatments. The present study has been approved by the local ethical committee.
Results
Six patients with the above-described clinical features were identified. From a histological point of view, they were 2 PDTCs, 2 PTCs, and 2 MTCs. Four patients underwent major surgery, performed by a multidisciplinary team, including a thoracic and an endocrine surgeon (patients #3–6). In the other two cases (patients #1 and #2), vascular and cardiac surgery expertise was also necessary because of neoplastic vascular and cardiac involvement requiring the use of extracorporeal circulation (ECC), cardioplegia, and vascular graft placement.
Presurgical evaluation
Extensive surgery was planned in all 6 patients: in 3 patients (#1, #2 and #3) as initial treatment and in the other 3 (#4, #5 and #6) after a previous initial treatment (total or near-total thyroidectomy). In particular, patient #4 presented a MTC (T1aN1aM0, stage III according to AJCC 8th edition), patient #5 a PDTC (T4NxM1, stage IV), and patient #6 a PTC (T2NxMx, stage I). In patients #3, #4, and #5, preoperative diagnosis was performed by fine-needle aspiration (FNA), while in two cases (#1 and #6), it was obtained by tru-cut [14] and in one case (#2) by ultrasound transbronchial needle aspiration (EBUS-TBNA).
Table 1 shows disease extension for each patient. Mediastinal lymph nodes were the most common site of disease (5 out of 6 patients), although laterocervical ones were also commonly involved (4 out of 6 patients). Interestingly, all patients experienced at least two sites of disease. Patient #5 also presented with distant metastases (lung).
A multidisciplinary evaluation was performed on all patients. In patients #1 and #2, radiotherapy and multikinase inhibitors’ treatments were not feasible due to a very high embolic risk; at the same time, their vascular invasion was considered life-threatening. In the other cases, surgery was indicated as the first therapeutic approach to achieve local disease control.
In their clinical history, all patients presented with co-morbidities: four patients experienced major cardiovascular disease (ischemic heart disease, heart failure, stroke and abdominal aorta aneurysm, and celiac tripod aneurysm), two were suffering from obesity with arterial hypertension, and one had undergone hysterectomy for uterine fibromatosis (Table 2). At the moment of surgery, all these comorbidities were considered clinically compensated thanks to their pharmacological treatment.
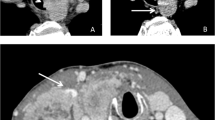
Moreover, at admission, 4 out of 6 patients suffered from symptoms related to their advanced disease. Patients #1 and #2 presented with mediastinal syndrome due to an extensive thrombus from the right jugular vein to the right atrium (Fig. 1A), and to an obstruction of the superior vena cava (Fig. 1B), respectively. In particular, they presented edema of neck, arms, and trunk, distended cervical and superficial chest collateral veins and cough. Patient #3, who was affected by MTC, presented with chronic secretory-type diarrhea, due to high levels of serum calcitonin, while patient #4 referred a severe and long-standing pain in the left laterocervical site.
Surgical treatments
At the time of surgery, the median age was 67.5 years (IQR 65.5–68.5, range 47–75). In all patients, surgery was performed through cervicotomy in order to remove any possible local recurrence and ensure an adequate bilateral neck dissection, and then through complete median sternotomy to isolate all mediastinal structures from the lymphadenopathy.
In patient #1, a radical thyroidectomy by peeling the tracheal surface and an extended cervical lymphadenectomy en-bloc with external right jugular vein and superior vena cava were performed, combined with cavo-atrial thromboarterectomy and vascular reconstruction with bovine pericardial patch (Fig. 2A). Likewise, patient #2 had a neoplastic infiltration of the anonymous artery and of the left brachiocephalic vein requiring vascular resection and reconstruction with Dacron grafts, and an endoluminal thrombus of the superior vena cava that was removed (Fig. 2B).
In 5 cases, we obtained a macroscopic complete resection confirmed by the histopathological examination (R0). In one case (patient #1), a macroscopic complete resection was not possible because of tracheal infiltration requiring surgical debulking by tracheal shaving (R2).
Wide lymph-node resection was performed in patient #2–6. In particular, 1 metastatic mediastinal lymph node (6 cm) with extranodal extension and pleural infiltration was surgically removed in patient #2, multiple and confluent cervical (biggest one of 5 cm) and mediastinal metastatic lymph node with wide extra nodal extension (biggest one of 3 cm) in patient #3, 24 metastatic cervical out of 50 surgically removed (biggest one of 2.0 cm) and 10 metastatic mediastinal out of 21 surgically removed (biggest one of 0.8 cm) lymph nodes in patient #4, 1 metastatic cervical of 4.0 cm out of 2 surgically removed and 1 mediastinal lymph node of 3.2 cm out of 3 surgically removed in patient #5, and 3 metastatic cervical out of 11 surgically removed (biggest one of 1.2 cm) and 1 of mediastinal lymph node of 2 cm out of 4 surgically removed in patient #6. Interestingly, in patients #4, #5, and #6, who have been already submitted to a previous surgery treatment, we observed a histological concordance between lesions surgically removed during initial and current treatment.
Adverse events
Surgical complications were observed in 4 out of 6 patients (Table 3). All 4 patients experienced typical thyroid surgery complications (i.e., hypoparathyroidism and laryngeal nerve injury with vocal cord paralysis). In particular, patients #3 and #6 had only hypoparathyroidism, patient #2 only right laryngeal nerve injury with vocal cord paralysis, and patient #1 both. As far as atypical thyroid surgery complications were concerned, pulmonary embolism was observed in patients #1 and #6.
Moreover, while patients #4 and #5 did not show any clinically significant complications, patients #1 and #2 developed multiple adverse events. In particular, patient #1 developed not only an asymptomatic pulmonary embolism revealed during radiological follow-up, but also a sternotomy dehiscence that required VAC-therapy and then a second surgery. Patient #2 had postoperative bleeding requiring gauze packing and a second surgery on the second postoperative day. During intensive care unit stay, the patient developed bilateral pneumonia with pleural effusion and respiratory failure requiring transitory tracheostomy.
No complications related to the vascular graft (e.g., dislocation) nor to the ECC (i.e., nervous system complications, such as bleeding, ischemia, and multiorgan failure, in particular renal failure) were observed. In this series, no intraoperative or perioperative mortality (30- and 90 day mortality, respectively) occurred.
Long-term follow-up
At study data lock (December 2022), patients #1–#4 did not develop the recurrence of symptoms observed before surgery. Patients #4 and #6 showed a biochemical persistence of the disease, and patients #1, #2, #3, and #5 showed a structural persistence of the disease. At data lock, 5 out of 6 patients were still alive after a median follow-up of 2.6 years (IQR 2.5–2.8, interval 2.3–3.6). Patient #1 died for reasons unrelated to the neoplastic disease after 2.5 years of good health conditions.
Figure 3 describes the therapies administered during follow-up. Patients #3 and #4 did not receive any further treatment after surgery. Radioiodine treatment was performed in 3 out of 6 patients (patients #1, #2 and #6), 4–6 months after surgery. Systemic therapy with multikinase inhibitor (MKI) (i.e. lenvatinib) was administered to 2 patients. Patient #1 started lenvatinib treatment 10 months after surgery for a tracheal recurrence, and also treated by endotracheal laser (twice, 10 months and 21 months after surgery), reaching local disease stabilization. Patient #5 started lenvatinib treatment for lung and a large mediastinal lymph-node metastasis that recurred 3 months after surgery, obtaining a partial response. In patient #2, a local treatment for tracheal recurrence was performed by placing an endotracheal stent.
Discussion
In the last 20 years, the incidence of TC has progressively increased, especially since TCs with a diameter less than 1 cm are more frequently diagnosed [1]. However, many authors observed that diagnosis of TCs with a larger diameter (> 4 cm) presented a significant increase too, as well as cases with extrathyroidal extension and lymph-node metastases [15, 16]. If the challenge is to avoid overtreatment in very low aggressive cases [17], in patients suffering from advanced TC, the challenge is to be ideally radical, weighting a potential significant morbidity [11].
We reported our experience in advanced TC cases treated with a multimodal approach, including surgical, radiometabolic, and systemic therapies. We confirmed the crucial role of surgery in treating advanced TC, beyond its well-systemized role in cases confined to the neck region [18]. In our clinical series, surgery had a fundamental role in: (1) reaching a prompt symptoms resolution related to the metastatic disease, (2) allowing adjuvant therapies, not feasible or probably ineffective without a radical surgery, and (3) improving survival, as a component of a multimodal treatment regimen. On the other hand, in our series, both typical and atypical thyroid surgical complications occurred.
At admission, most of our patients presented symptoms related to the metastatic disease and, in particular, two of them mediastinal syndrome. As recently reviewed, mediastinal syndrome is very rare in TC, with a very poor prognosis [19]. Since 1879 onwards, about 60 cases of TCs with major vascular infiltration have been reported, and mediastinal vascular infiltration has been described in less than 30 cases [19,20,21]. In our series, surgery allowed a prompt symptoms resolution related to vascular involvement and, during a median follow-up of 2.6 years, none of our patients developed symptom recurrence, confirming that surgery should always be evaluated in case of vascular infiltration or symptomatic patients [19]. Likewise, a massive tumor debulking should be taken into consideration in locally advanced MTC patients suffering from chronic diarrhea to reduce the complaints [22], albeit new target therapies have been demonstrated to be effective, too [23]. In our case, diarrhea disappeared immediately after surgery, without recurrence during a 3 year follow-up.
Beyond symptomatic relief, local disease control is one of the main oncologic targets of an enlarged surgery [11]. In a recent report of series including 153 DTCs with invasion of subcutaneous soft tissues, recurrent laryngeal nerve, larynx, trachea or esophagus, 5 year disease-specific survival, as well as locoregional recurrence did not differ between cases with complete resection (R0) and with positive margin of resection (R1) [24]. Furthermore, regional eradication or debulking of the tumor mass, resulting in macroscopically radical surgery or in minimal residual disease, can allow other therapeutic options. In particular, two of our patients after surgery could ultimately initiate treatments with MKIs that were not feasible before surgery because of the extensive venous thrombosis. Lenvatinib, cabozantinib, and other MKIs are strong inhibitors of vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR) [25, 26]. Since VEGFR regulates endothelial function, it is not surprising that its inhibition predisposes to platelet activation and thrombosis [27, 28]. Bai et al. carried out an extensive meta-analysis about the risk of thromboembolic events during MKIs treatment in advanced TC, and observed an increased risk of arterial thromboembolic events [29]. In SELECT trial, a phase III trial evaluating progression-free survival (PFS), response rate, overall survival (OS), and toxicity of lenvatinib [30], venous thromboembolic events were observed in 5.4% of cases in the lenvatinib arm, and in 3.8% of cases in the placebo arm. Likewise, in EXAM trial, a phase III trial evaluating PFS, OS, objective response rate, and safety of cabozantinib, pulmonary embolism was observed in 2.3% of patients treated with cabozantinib, and in none of the patients in the placebo arm [31]. In line with this evidence, the multidisciplinary team decided not to start MKI in the case of such big thrombosis, while it was possible after its debulking.
Regional disease eradication or debulking could reinforce the effect of following systemic therapies, whose efficacy depends on tumor burden. An updated analysis of SELECT trial by Gianoukakis et al. showed different duration of response induced by lenvatinib according to the baseline diseases burden [32]. Radioiodine efficacy too has been related to tumor burden [33]. Song et al. showed that both biochemical and structural responses achieved after radioiodine treatment were poorer in patients with higher disease burden [34]. In our clinical series, some patients performed effective radioiodine treatments after surgery that was not decisive, but at least stabilized the disease that, in our opinion, is anyway a good clinical result in this type of patients.
In our series, despite their advanced stage and multiple comorbidities, we did not observe any perioperative deaths or any cancer-related deaths during a median follow-up of 2.6 years. Our results are in line with those of other reported series. Marcy et al. described 9 cases of TC characterized by neoplastic venous obstruction, drawn from a database of 1171 patients [35]. They argued that very invasive multimodal treatment including surgery should be performed to improve survival [35]. The same conclusion was reached by Hyer et al., who studied a series of 5 patients with venous obstruction [36]. However, this extensive surgery is not devoid of complications, both typical and atypical for endocrine surgery. About the former, 4 patients developed hypoparathyroidism and/or laryngeal nerve injury with vocal cord paralysis. However, it is well known that the risk of typical complications for endocrine surgery, such as hypoparathyroidism, is influenced by the extent of neck dissection [37, 38]. About the latter, 2 patients developed pulmonary embolism and 1 patient sternotomy dehiscence. Even though the incidence of deep venous thrombosis and pulmonary embolism is low after conventional thyroid surgery [39], in patients with diffuse neoplastic thrombosis or undergoing aggressive thoracic lymphoadenectomy the incidence is not negligible [12, 40]. All these information and evidences must be well known by the multidisciplinary team that, for these reasons, should include an expert endocrinologist in advanced TC, an endocrine surgeon with a great experience of TC and other highly experienced specialists, such as thoracic, vascular, and otolaryngologist surgeons, tracheobroncoscopist, anesthetists, and others specialists according to the extension of the disease and comorbidities of the patient.
Conclusions
Our experience in the treatment of very advanced TCs, particularly at local level, shows the effectiveness of an extensive surgery performed by a multidisciplinary team in controlling symptoms, allowing adjuvant therapies, and improving the survival of patients whose cases would otherwise be very difficult to manage. The planning of a well-defined therapeutic strategy by a multidisciplinary team and the simultaneous presence at the operating table of expert surgeons of different disciplines determined a favorable outcome. Still relatively young patients can gain significant benefits from this therapeutic approach, mainly a longer survival and a chronicity of the disease with whom they cohabit for a long time. However, this type of surgery is possible, and should be performed, only in expert tertiary referral centers for the management of TC.
References
Kitahara CM, Sosa JA (2016) The changing incidence of thyroid cancer. Nat Rev Endocrinol 12:646–653. https://doi.org/10.1038/nrendo.2016.110
Fagin JA, Wells SA (2016) Biologic and clinical perspectives on thyroid cancer. N Engl J Med 375:1054–1067. https://doi.org/10.1056/NEJMra1501993
Prete A, Borges de Souza P, Censi S et al (2020) Update on fundamental mechanisms of thyroid cancer. Front Endocrinol 11:102. https://doi.org/10.3389/fendo.2020.00102
Cabanillas ME, McFadden DG, Durante C (2016) Thyroid cancer. Lancet 388:2783–2795. https://doi.org/10.1016/S0140-6736(16)30172-6
Prete A, Matrone A, Gambale C et al (2021) Poorly differentiated and anaplastic thyroid cancer: insights into genomics. Microenviron New Drugs Cancers 13:3200. https://doi.org/10.3390/cancers13133200
Durante C, Haddy N, Baudin E et al (2006) Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91:2892–2899. https://doi.org/10.1210/jc.2005-2838
Mirian C, Grønhøj C, Jensen DH et al (2018) Trends in thyroid cancer: retrospective analysis of incidence and survival in Denmark 1980–2014. Cancer Epidemiol 55:81–87. https://doi.org/10.1016/j.canep.2018.05.009
de la Fouchardière C, Decaussin-Petrucci M, Berthiller J et al (2018) Predictive factors of outcome in poorly differentiated thyroid carcinomas. Eur J Cancer 92:40–47. https://doi.org/10.1016/j.ejca.2017.12.027
Matrone A, Gambale C, Prete A et al (2020) Impact of advanced age on the clinical presentation and outcome of sporadic medullary thyroid carcinoma. Cancers 13:94. https://doi.org/10.3390/cancers13010094
Pacini F, Basolo F, Bellantone R et al (2018) Italian consensus on diagnosis and treatment of differentiated thyroid cancer: joint statements of six Italian societies. J Endocrinol Invest 41:849–876. https://doi.org/10.1007/s40618-018-0884-2
Russell MD, Kamani D, Randolph GW (2020) Modern surgery for advanced thyroid cancer: a tailored approach. Gland Surg 9:S105–S119. https://doi.org/10.2103/gs.2019.12.16
Porterfield JR, Cassivi SD, Wigle DA et al (2009) Thoracic metastasectomy for thyroid malignancies. Eur J Cardiothorac Surg 36:155–158. https://doi.org/10.1016/j.ejcts.2008.12.055
Raue F, Frank-Raue K (2016) Thyroid cancer: risk-stratified management and individualized therapy. Clin Cancer Res 22:5012–5021. https://doi.org/10.1158/1078-0432.CCR-16-0484
Matrone A, De Napoli L, Torregrossa L et al (2022) Core needle biopsy can early and precisely identify large thyroid masses. Front Oncol 12:854755. https://doi.org/10.3389/fonc.2022.854755
Morris LGT, Myssiorek D (2010) Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 200:454–461. https://doi.org/10.1016/j.amjsurg.2009.11.008
Dahlberg J, Adok C, Bümming P et al (2021) Incidence, detection and outcome of differentiated thyroid cancer in Western Sweden. BJS Open 5:099. https://doi.org/10.1093/bjsopen/zrab099
Molinaro E, Campopiano MC, Pieruzzi L et al (2020) Active surveillance in papillary thyroid microcarcinomas is feasible and safe: experience at a single Italian center. J Clin Endocrinol Metab 105:e172-180. https://doi.org/10.1210/clinem/dgz113
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133. https://doi.org/10.1089/thy.2015.0020
Chen W, Lei J, Wang Y et al (2021) Case report: superior vena cava resection and reconstruction for invasive thyroid cancer: report of three cases and literature review. Front Surg 8:644605. https://doi.org/10.3389/fsurg.2021.644605
Shirah BH, Shirah HA, Al Hassan AK, Elnour AE (2018) Retrosternal metastatic papillary thyroid cancer causing superior vena cava syndrome: a very rare presentation. Ann Vasc Surg 46:368.e9-368.e12. https://doi.org/10.1016/j.avsg.2017.08.025
Xu MS, Li J, Wiseman SM (2019) Major vessel invasion by thyroid cancer: a comprehensive review. Expert Rev Anticancer Ther 19:191–203. https://doi.org/10.1080/14737140.2019.1559059
Dadu R, Hu MI, Cleeland C et al (2015) Efficacy of the natural clay, calcium aluminosilicate anti-diarrheal, in reducing medullary thyroid cancer-related diarrhea and its effects on quality of life: a pilot study. Thyroid 25:1085–1090. https://doi.org/10.1089/thy.2015.0166
Wirth LJ, Sherman E, Robinson B et al (2020) Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med 383:825–835. https://doi.org/10.1056/NEJMoa2005651
Wang LY, Nixon IJ, Patel SG et al (2016) Operative management of locally advanced, differentiated thyroid cancer. Surgery 160:738–746. https://doi.org/10.1016/j.surg.2016.04.027
Matrone A, Valerio L, Pieruzzi L et al (2017) Protein kinase inhibitors for the treatment of advanced and progressive radiorefractory thyroid tumors: from the clinical trials to the real life. Best Pract Res Clin Endocrinol Metab 31:319–334. https://doi.org/10.1016/j.beem.2017.06.001
Matrone A, Prete A, Nervo A et al (2021) Lenvatinib as a salvage therapy for advanced metastatic medullary thyroid cancer. J Endocrinol Invest 44:2139–2151. https://doi.org/10.1007/s40618-020-01491-3
Touyz RM, Herrmann SMS, Herrmann J (2018) Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J Am Soc Hypertens 12:409–425. https://doi.org/10.1016/j.jash.2018.03.008
Dobbin SJH, Cameron AC, Petrie MC et al (2018) Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors. Heart 104:1995–2002. https://doi.org/10.1136/heartjnl-2018-313726
Bai Y, Li J-Y, Li J et al (2019) Risk of venous and arterial thromboembolic events associated with tyrosine kinase inhibitors in advanced thyroid cancer: a meta-analysis and systematic review. Oncotarget 10:4205–4212. https://doi.org/10.1863/oncotarget.24599
Schlumberger M, Tahara M, Wirth LJ et al (2015) Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621–630. https://doi.org/10.1056/NEJMoa1406470
Elisei R, Schlumberger MJ, Müller SP et al (2013) Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31:3639–3646. https://doi.org/10.1200/JCO.2012.48.4659
Gianoukakis AG, Dutcus CE, Batty N et al (2018) Prolonged duration of response in lenvatinib responders with thyroid cancer. Endocr Relat Cancer 25:699–704. https://doi.org/10.1530/ERC-18-0049
Wang R, Zhang Y, Tan J et al (2017) Analysis of radioiodine therapy and prognostic factors of differentiated thyroid cancer patients with pulmonary metastasis: an 8-year retrospective study. Medicine 96:e6809. https://doi.org/10.1097/MD.0000000000006809
Song H-J, Qiu Z-L, Shen C-T et al (2015) Pulmonary metastases in differentiated thyroid cancer: efficacy of radioiodine therapy and prognostic factors. Eur J Endocrinol 173:399–408. https://doi.org/10.1530/EJE-15-0296
Marcy P-YR, Thariat J, Bozec A et al (2009) Venous obstruction of thyroid malignancy origin: the Antoine Lacassagne institute experience. World J Surg Oncol 7:40. https://doi.org/10.1186/1477-7819-7-40
Hyer SL, Dandekar P, Newbold K et al (2008) Thyroid cancer causing obstruction of the great veins in the neck. World J Surg Oncol 6:36. https://doi.org/10.1186/1477-7819-6-36
Dedivitis RA, Aires FT, Cernea CR (2017) Hypoparathyroidism after thyroidectomy: prevention, assessment and management. Curr Opin Otolaryngol Head Neck Surg 25:142–146. https://doi.org/10.1097/MOO.0000000000000346
Meola A, Vignali E, Matrone A et al (2018) Efficacy and safety of long-term management of patients with chronic post-surgical hypoparathyroidism. J Endocrinol Invest 41:1221–1226. https://doi.org/10.1007/s40618-018-0857-5
Roy M, Rajamanickam V, Chen H, Sippel R (2010) Is DVT prophylaxis necessary for thyroidectomy and parathyroidectomy? Surgery 148:1163–1168. https://doi.org/10.1016/j.surg.2010.09.013
Kobayashi T, Ogura K, Nishizawa K et al (2004) Successful recovery from a massive pulmonary artery tumor embolism occurring during surgery for renal cell carcinoma. Int J Urol 11:114–116. https://doi.org/10.1111/j.1442-2042.2004.00743.x
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. This study has been supported by grants to R.E. from Associazione Italiana per la Ricerca sul Cancro (AIRC, Investigator grant 2018, project code 21790) and Agenzia Italiana del Farmaco (AIFA, project code AIFA-2016- 02365049).
Author information
Authors and Affiliations
Contributions
Conceptualization, AP, EP, EM, and RE; data curation, AP, EP, and EM; formal analysis, AP, EP, and EM; investigation, AP, EP, EM, LB, and MF; methodology AP, EP, EM, MF, and GS; supervision, ML, RE, and G M; writing—original draft, AP, EP, and EM; writing—review and editing, AP, EP, and VA; visualization: FB, CEA, and EM. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethical approval
The present study has been approved by the local ethical committee.
Informed consent
All patients signed an informed consent for the use of their clinical and biochemical data for research purposes. They also signed an informed consent to proceed with the suggested interventional treatments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prete, A., Pieroni, E., Marrama, E. et al. Management of patients with extensive locally advanced thyroid cancer: results of multimodal treatments. J Endocrinol Invest 47, 1165–1173 (2024). https://doi.org/10.1007/s40618-023-02234-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02234-w