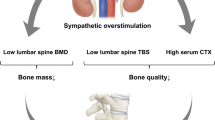
Abstract
Purpose
Despite the potentially destructive effect of sympathetic activity on bone metabolism, its impact on bone microarchitecture, a key determinant of bone quality, has not been thoroughly investigated. This study aims to evaluate the impact of sympathetic activity on bone microarchitecture and bone strength in patients with pheochromocytoma and paraganglioma (PPGL).
Methods
A cross-sectional study was conducted in 38 PPGL patients (15 males and 23 females). Bone turnover markers serum procollagen type 1 N-terminal propeptide (P1NP) and β-carboxy-terminal crosslinked telopeptide of type 1 collagen (β-CTX) were measured. 24-h urinary adrenaline (24hUE) and 24-h urinary norepinephrine levels (24hUNE) were measured to indicate sympathetic activity. High-resolution peripheral quantitative computed tomography (HR-pQCT) was conducted to evaluate bone microarchitecture in PPGL patients and 76 age-, sex-matched healthy controls (30 males and 46 females). Areal bone mineral density (aBMD) was measured by dual-energy X-ray absorptiometry (DXA) simultaneously.
Results
PPGL patients had a higher level of β-CTX. HR-pQCT assessment revealed that PPGL patients had notably thinner and more sparse trabecular bone (decreased trabecular number and thickness with increased trabecular separation), significantly decreased volume BMD (vBMD), and bone strength at both the radius and tibia compared with healthy controls. The deterioration of Tt.vBMD, Tb.Sp, and Tb.1/N.SD was more pronounced in postmenopausal patients compared with the premenopausal subjects. Moreover, subjects in the highest 24hUNE quartile (Q4) showed markedly lower Tb.N and higher Tb.Sp and Tb.1/N.SD at the tibia than those in the lowest quartile (Q1). Age-related bone loss was also exacerbated in PPGL patients to a certain extent.
Conclusions
PPGL patients had significantly deteriorated bone microarchitecture and strength, especially in the trabecular bone, with an increased bone resorption rate. Our findings provide clinical evidence that sympathetic overstimulation may serve as a secondary cause of osteoporosis, especially in subjects with increased sympathetic activity.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding authors. The data are not publicly available due to privacy or ethical restrictions.
References
Consensus development conference (1993) Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94:646–650. https://doi.org/10.1016/0002-9343(93)90218-e
Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1994, 843, 1–129.
Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141. https://doi.org/10.1002/jbmr.5650090802
Watts NB (2004) Fundamentals and pitfalls of bone densitometry using dual-energy X-ray absorptiometry (DXA). Osteoporos Int 15:847–854. https://doi.org/10.1007/s00198-004-1681-7
Kanis JA, Cooper C, Rizzoli R, Reginster JY (2019) Scientific advisory board of the european society for, c.; economic aspects of, O.; the committees of scientific, A.; national societies of the international osteoporosis, F. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30:3–44. https://doi.org/10.1007/s00198-018-4704-5
Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA, Hochberg MC, Vogt MT, Orwoll ES (2005) Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 90:2787–2793. https://doi.org/10.1210/jc.2004-1568
Vilayphiou N, Boutroy S, Szulc P, van Rietbergen B, Munoz F, Delmas PD, Chapurlat R (2011) Finite element analysis performed on radius and tibia HR-pQCT images and fragility fractures at all sites in men. J Bone Miner Res 26:965–973. https://doi.org/10.1002/jbmr.297
Geusens P, Chapurlat R, Schett G, Ghasem-Zadeh A, Seeman E, de Jong J, van den Bergh J (2014) High-resolution in vivo imaging of bone and joints: a window to microarchitecture. Nat Rev Rheumatol 10:304–313. https://doi.org/10.1038/nrrheum.2014.23
Metcalf LM, Dall’Ara E, Paggiosi MA, Rochester JR, Vilayphiou N, Kemp GJ, McCloskey EV (2018) Validation of calcaneus trabecular microstructure measurements by HR-pQCT. Bone 106:69–77. https://doi.org/10.1016/j.bone.2017.09.013
Edwards MH, Robinson DE, Ward KA, Javaid MK, Walker-Bone K, Cooper C, Dennison EM (2016) Cluster analysis of bone microarchitecture from high resolution peripheral quantitative computed tomography demonstrates two separate phenotypes associated with high fracture risk in men and women. Bone 88:131–137. https://doi.org/10.1016/j.bone.2016.04.025
Tsai JN, Uihlein AV, Burnett-Bowie SM, Neer RM, Derrico NP, Lee H, Bouxsein ML, Leder BZ (2016) Effects of two years of teriparatide, denosumab, or both on bone microarchitecture and strength (DATA-HRpQCT study). J Clin Endocrinol Metab 101:2023–2030. https://doi.org/10.1210/jc.2016-1160
Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M et al (2005) Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520. https://doi.org/10.1038/nature03398
Bonnet N, Laroche N, Vico L, Dolleans E, Benhamou CL, Courteix D (2006) Dose effects of propranolol on cancellous and cortical bone in ovariectomized adult rats. J Pharmacol Exp Ther 318:1118–1127. https://doi.org/10.1124/jpet.106.105437
Kondo H, Togari A (2011) Continuous treatment with a low-dose beta-agonist reduces bone mass by increasing bone resorption without suppressing bone formation. Calcif Tissue Int 88:23–32. https://doi.org/10.1007/s00223-010-9421-9
Schlienger RG, Kraenzlin ME, Jick SS, Meier CR (2004) Use of beta-blockers and risk of fractures. JAMA 292:1326–1332. https://doi.org/10.1001/jama.292.11.1326
Veldhuis-Vlug AG, Tanck MW, Limonard EJ, Endert E, Heijboer AC, Lips P, Fliers E, Bisschop PH (2015) The effects of beta-2 adrenergic agonist and antagonist on human bone metabolism: a randomized controlled trial. Bone 71:196–200. https://doi.org/10.1016/j.bone.2014.10.024
de Vries F, Pouwels S, Bracke M, Leufkens HG, Cooper C, Lammers JW, van Staa TP (2007) Use of beta-2 agonists and risk of hip/femur fracture: a population-based case-control study. Pharmacoepidemiol Drug Saf 16:612–619. https://doi.org/10.1002/pds.1318
Nolting S, Bechmann N, Taieb D, Beuschlein F, Fassnacht M, Kroiss M, Eisenhofer G, Grossman A, Pacak K (2022) Personalized management of pheochromocytoma and paraganglioma. Endocr Rev 43:199–239. https://doi.org/10.1210/endrev/bnab019
Kim BJ, Kwak MK, Ahn SH, Kim H, Lee SH, Song KH, Suh S, Kim JH, Koh JM (2017) Lower bone mass and higher bone resorption in pheochromocytoma: importance of sympathetic activity on human bone. J Clin Endocrinol Metab 102:2711–2718. https://doi.org/10.1210/jc.2017-00169
Veldhuis-Vlug AG, El Mahdiui M, Endert E, Heijboer AC, Fliers E, Bisschop PH (2012) Bone resorption is increased in pheochromocytoma patients and normalizes following adrenalectomy. J Clin Endocrinol Metab 97:E2093-2097. https://doi.org/10.1210/jc.2012-2823
Yokomoto-Umakoshi M, Umakoshi H, Sakamoto R, Fukumoto T, Ogata M, Nakano Y, Iwahashi N, Kaneko H, Mizoguchi N, Hattori A et al (2021) Role of deteriorated bone quality in the development of osteoporosis in pheochromocytoma and paraganglioma. Bone 142:115607. https://doi.org/10.1016/j.bone.2020.115607
Yu F, Xu Y, Hou Y, Lin Y, Jiajue R, Jiang Y, Wang O, Li M, Xing X, Zhang L et al (2020) Age-, site-, and sex-specific normative centile curves for hr-pqct-derived microarchitectural and bone strength parameters in a chinese mainland population. J Bone Miner Res 35:2159–2170. https://doi.org/10.1002/jbmr.4116
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90:6508–6515. https://doi.org/10.1210/jc.2005-1258
Whittier DE, Boyd SK, Burghardt AJ, Paccou J, Ghasem-Zadeh A, Chapurlat R, Engelke K, Bouxsein ML (2020) Guidelines for the assessment of bone density and microarchitecture in vivo using high-resolution peripheral quantitative computed tomography. Osteoporos Int 31:1607–1627. https://doi.org/10.1007/s00198-020-05438-5
Li M, Lv F, Zhang Z, Deng W, Li Y, Deng Z, Jiang Y, Wang O, Xing X, Xu L et al (2016) Establishment of a normal reference value of parathyroid hormone in a large healthy Chinese population and evaluation of its relation to bone turnover and bone mineral density. Osteoporos Int 27:1907–1916. https://doi.org/10.1007/s00198-015-3475-5
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14:595–608. https://doi.org/10.1016/8756-3282(93)90081-k
Fonseca H, Moreira-Goncalves D, Coriolano HJ, Duarte JA (2014) Bone quality: the determinants of bone strength and fragility. Sports Med 44:37–53. https://doi.org/10.1007/s40279-013-0100-7
Kim BJ, Kwak MK, Kim JS, Lee SH, Koh JM (2018) Higher sympathetic activity as a risk factor for skeletal deterioration in pheochromocytoma. Bone 116:1–7. https://doi.org/10.1016/j.bone.2018.06.023
Amin S, Khosla S (2012) Sex- and age-related differences in bone microarchitecture in men relative to women assessed by high-resolution peripheral quantitative computed tomography. J Osteoporos. https://doi.org/10.1155/2012/129760
Bonnet N, Gadois C, McCloskey E, Lemineur G, Lespessailles E, Courteix D, Benhamou CL (2007) Protective effect of beta blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone 40:1209–1216. https://doi.org/10.1016/j.bone.2007.01.006
Szulc P, Boutroy S, Vilayphiou N, Chaitou A, Delmas PD, Chapurlat R (2011) Cross-sectional analysis of the association between fragility fractures and bone microarchitecture in older men: the STRAMBO study. J Bone Miner Res 26:1358–1367. https://doi.org/10.1002/jbmr.319
Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD (2007) Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res 22:425–433. https://doi.org/10.1359/jbmr.061206
Kawalilak CE, Johnston JD, Olszynski WP, Kontulainen SA (2014) Characterizing microarchitectural changes at the distal radius and tibia in postmenopausal women using HR-pQCT. Osteoporos Int 25:2057–2066. https://doi.org/10.1007/s00198-014-2719-0
Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E (2010) Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet 375:1729–1736. https://doi.org/10.1016/S0140-6736(10)60320-0
Reid IR (2004) Leptin deficiency–lessons in regional differences in the regulation of bone mass. Bone 34:369–371. https://doi.org/10.1016/j.bone.2003.11.007
Samelson EJ, Broe KE, Xu H, Yang L, Boyd S, Biver E, Szulc P, Adachi J, Amin S, Atkinson E et al (2019) Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): a prospective study. Lancet Diabetes Endocrinol 7:34–43. https://doi.org/10.1016/s2213-8587(18)30308-5
Yokomoto-Umakoshi M, Umakoshi H, Fukumoto T, Matsuda Y, Nagata H, Ogata M, Kawate H, Miyazawa T, Sakamoto R, Ogawa Y et al (2020) Pheochromocytoma and paraganglioma: an emerging cause of secondary osteoporosis. Bone 133:115221. https://doi.org/10.1016/j.bone.2020.115221
Farr JN, Charkoudian N, Barnes JN, Monroe DG, McCready LK, Atkinson EJ, Amin S, Melton LJ, Joyner MJ, Khosla S (2012) Relationship of sympathetic activity to bone microstructure, turnover, and plasma osteopontin levels in women. J Clin Endocrinol Metab 97:4219–4227. https://doi.org/10.1210/jc.2012-2381
Acknowledgements
The authors express heartfelt thanks to all the patients and the healthy controls for their participation in the study.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 82270938); the Chinese National Key Technology R&D Program, Ministry of Science and Technology (2021YFC2501700); and the Chinese Academy of Medical Sciences-CAMS Innovation Fund for Medical Sciences (CIFMS-2021-I2M-1–002).
Author information
Authors and Affiliations
Contributions
AT and WX designed the study and revised the manuscript. WQ analyzed the data and drafted the manuscript. LC, RJ, QP, YC, YJ, OW, Ml, and XX collected clinical information of patients. WQ, AT, and WX are responsible for the integrity of the data analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Department of Scientific Research, PUMCH (ZS-1689).
Informed consent
Informed consent was obtained from the parents of all individual participants included.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, W., Cui, L., Jiajue, R. et al. Deteriorated bone microarchitecture caused by sympathetic overstimulation in pheochromocytoma and paraganglioma. J Endocrinol Invest 47, 843–856 (2024). https://doi.org/10.1007/s40618-023-02198-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02198-x