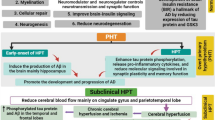
Abstract
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by loss of dopaminergic neurons. Dopaminergic system is interconnected with the hypothalamic–pituitary–thyroid axis. Dopamine (DA) upregulates thyrotropin releasing hormone (TRH) while downregulating thyroid stimulating hormone (TSH) and thyroid hormones. Moreover, TRH stimulates DA release. PD is associated with impaired regulation of TSH and thyroid hormones (TH) levels, which in turn associate with severity and different subtypes of PD, while levodopa and bromocriptine treatment can interfere with hypothalamic–pituitary–thyroid axis. Thyroid disturbances, including hypothyroidism, Hashimoto’s thyroiditis (HT), hyperthyroidism and Graves’ disease (GD) not only increase the risk of PD but also share some clinical signs with PD. Also, several genes including RASD2, WSB1, MAPT, GIRK2, LRRK2 and gene products like neurotensin and NOX/DUOX affect the risk for both PD and thyroid disease. Hypothyroidism is associated with obesity, hypercholesterolemia, anemia and altered cerebral blood flow which are associated with PD pathology. Herein we provide a comprehensive view on the association between PD and thyroid hormones regulation and dysregulations, hoping to provide new avenues towards targeted treatment of PD. We performed a comprehensive search in literature using Pubmed and Scopus, yielding to a total number of 36 original articles that had addressed the association between thyroid hormone disorders and PD.


Similar content being viewed by others
References
De Virgilio A, Greco A, Fabbrini G, Inghilleri M, Rizzo MI, Gallo A et al (2016) Parkinson's disease: autoimmunity and neuroinflammation. Autoimmun Rev 15(10):1005–1011
Reich SG, Savitt JM (2019) Parkinson's disease. Med Clin N Am 103(2):337–350
Postuma RB, Berg D (2016) Advances in markers of prodromal Parkinson disease. Nat Rev Neurol 12:622
Beaulieu JM, Espinoza S, Gainetdinov RR (2015) Dopamine receptors—IUPHAR review 13. Br J Pharmacol 172(1):1–23
Jaber M, Robinson SW, Missale C, Caron MG (1996) Dopamine receptors and brain function. Neuropharmacology 35(11):1503–1519
Boyd KN, Mailman RB (2012) Dopamine receptor signaling and current and future antipsychotic drugs. Handb Exp Pharmacol 212:53–86
Gaum PM, Gube M, Esser A, Schettgen T, Quinete N, Bertram J et al (2019) Depressive symptoms after PCB exposure: hypotheses for underlying pathomechanisms via the thyroid and dopamine system. Int J Environ Res Public Health 16(6):950
Bavarsad K, Hosseini M, Hadjzadeh MA, Sahebkar A (2019) The effects of thyroid hormones on memory impairment and Alzheimer's disease. J Cell Physiol 234(9):14633–14640
Aranda A, Alonso-Merino E, Zambrano A (2013) Receptors of thyroid hormones. Pediatr Endocrinol Rev PER 11(1):2–13
Choi SM, Kim BC, Choi KH, Nam TS, Kim JT, Lee SH et al (2014) Thyroid status and cognitive function in euthyroid patients with early Parkinson's disease. Dement Geriatr Cogn Disord 38(3–4):178–185
Li X, Sundquist J, Sundquist K (2012) Subsequent risks of Parkinson disease in patients with autoimmune and related disorders: a nationwide epidemiological study from Sweden. Neurodegener Dis 10(1–4):277–284
Stang A (2010) Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Ben-Jonathan N (1985) Dopamine: a prolactin-inhibiting hormone. Endocr Rev 6(4):564–589
Bissonette G, Roesch M (2016) Development and function of the midbrain dopamine system: what we know and what we need to. Genes Brain Behav 15(1):62–73
Sanjari Moghaddam H, Zare-Shahabadi A, Rahmani F, Rezaei N (2017) Neurotransmission systems in Parkinson’s disease. Rev Neurosci 8(5):509–536
Cantuti-Castelvetri I, Hernandez LF, Keller-McGandy CE, Kett LR, Landy A, Hollingsworth ZR et al (2010) Levodopa-induced dyskinesia is associated with increased thyrotropin releasing hormone in the dorsal striatum of hemi-parkinsonian rats. PLoS ONE 5(11):e13861
Daimon CM, Chirdon P, Maudsley S, Martin B (2013) The role of thyrotropin releasing hormone in aging and neurodegenerative diseases. Am J Alzheimer's Dis (Columbia, Mo) 1(1)
Zheng C, Chen G, Tan Y, Zeng W, Peng Q, Wang J et al (2018) TRH analog, taltirelin improves motor function of hemi-PD rats without inducing dyskinesia via sustained dopamine stimulating effect. Front Cell Neurosci 12:417
Singh O, Pradhan DR, Nagalakashmi B, Kumar S, Mitra S, Sagarkar S et al (2019) Thyrotropin-releasing hormone (TRH) in the brain and pituitary of the teleost, Clarias batrachus and its role in regulation of hypophysiotropic dopamine neurons. J Comp Neurol 527(6):1070–1101
Zheng C, Chen G, Tan Y, Zeng W, Peng Q, Wang J et al (2018) TRH analog, taltirelin protects dopaminergic neurons from neurotoxicity of MPTP and rotenone. Front Cell Neurosci 12:485
Lambeir AM (2011) Interaction of prolyl oligopeptidase with alpha-synuclein. CNS Neurol Disord Drug Targets 10(3):349–354
Goldstein J, Perello M, Nillni EA (2007) Preprothyrotropin-releasing hormone 178–199 affects tyrosine hydroxylase biosynthesis in hypothalamic neurons. J Mol Neurosci 31(1):69–82
Nillni EA, Aird F, Seidah NG, Todd RB, Koenig JI (2001) PreproTRH178–199 and two novel peptides (pFQ7 and pSE14) derived from its processing, which are produced in the paraventricular nucleus of the rat hypothalamus, are regulated during suckling**This work was supported by the National Science Foundation (Grant No. IBN-9507952 to E.A.N.). Endocrinology 142(2):896–906
Nillni EA (2010) Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front Neuroendocrinol 31(2):134–156
Haugen BR (2009) Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab 23(6):793–800
Pereira JC Jr, Pradella-Hallinan M, Pessoa HDL (2010) Imbalance between thyroid hormones and the dopaminergic system might be central to the pathophysiology of restless legs syndrome: a hypothesis. Clinics 65(5):547–554
Feek C, Sawers J, Brown N, Seth J, Irvine W, Toft A (1980) Influence of thyroid status on dopaminergic inhibition of thyrotropin and prolactin secretion: evidence for an additional feedback mechanism in the control of thyroid hormone secretion. J Clin Endocrinol Metab 51(3):585–589
Filippi L, Pezzati M, Cecchi A, Poggi C (2006) Dopamine infusion: a possible cause of undiagnosed congenital hypothyroidism in preterm infants. Pediatr Crit Care Med 7(3):249–251
Lestingi L, Bonifati V, Stocchi F, Antonozzi I, Meco G (1992) TRH test and the continuous dopaminergic stimulation in complicated Parkinson’s disease. Eur Neurol 32(2):65–69
Annunziato L, Di Renzo G, Schettini G, Lombardi G, Scopacasa F, Scapagnini U et al (1979) Lack of evidence for an inhibitory role played by tuberoinfundibular dopaminergic neurons on TSH secretion in the rat. Neuroendocrinology 28(6):435–441
Williams FL, Ogston SA, van Toor H, Visser TJ, Hume R (2005) Serum thyroid hormones in preterm infants: associations with postnatal illnesses and drug usage. J Clin Endocrinol Metab 90(11):5954–5963
Kobusiak-Prokopowicz M, Ściborski K, Mysiak A (2012) Effect of intravenous dopamine infusion on pituitary and thyroid function and on nephroprotection. Pol Arch Med Wewn 122(3):82–88
Mori T, Yokota T, Akamizu T, Inoue D, Miyamoto M, Kosugi S et al (1988) Significance of serum thyrotropin and plasma dopamine concentration in the regulation of thyroid function in elderly subjects. Endocrinol Jpn 35(3):469–476
Mouri A, Hoshino Y, Narusawa S, Ikegami K, Mizoguchi H, Murata Y et al (2014) Thyrotoropin receptor knockout changes monoaminergic neuronal system and produces methylphenidate-sensitive emotional and cognitive dysfunction. Psychoneuroendocrinology 48:147–161
Maayan ML, Sellitto RV, Volpert EM (1986) Dopamine and L-dopa: inhibition of thyrotropin-stimulated thyroidal thyroxine release. Endocrinology 118(2):632–636
Ekmen S, Degirmencioglu H, Uras N, Oncel MY, Sari FN, Canpolat FE et al (2015) Effect of dopamine infusion on thyroid hormone tests and prolactin levels in very low birth weight infants. J Matern Fetal Neonatal Med 28(8):924–927
Hassan WA, Aly MS, Rahman TA, Shahat AS (2013) Impact of experimental hypothyroidism on monoamines level in discrete brain regions and other peripheral tissues of young and adult male rats. Int J Dev Neurosci 31(4):225–233
Overstreet DH, Crocker AD, Lawson CA, McIntosh GH, Crocker JM (1984) Alterations in the dopaminergic system and behaviour in rats reared on iodine-deficient diets. Pharmacol Biochem Behav 21(4):561–565
Puymirat J, Faivre-Bauman A, Barret A, Loudes C, Tixier-Vidal A (1985) Does triiodothyronine influence the morphogenesis of fetal mouse mesencephalic dopaminergic neurons cultured in chemically defined medium? Dev Brain Res 23(2):315–317
Reymond MJ, Benotto W, Lemarchand-Beraud T (1987) The secretory activity of the tuberoinfundibular dopaminergic neurons is modulated by the thyroid status in the adult rat: consequence on prolactin secretion. Neuroendocrinology 46(1):62–68
Shimokawa N, Yousefi B, Morioka S, Yamaguchi S, Ohsawa A, Hayashi H et al (2014) Altered cerebellum development and dopamine distribution in a rat genetic model with congenital hypothyroidism. J Neuroendocrinol 26(3):164–175
Oh-Nishi A, Saji M, Furudate SI, Suzuki N (2005) Dopamine D2-like receptor function is converted from excitatory to inhibitory by thyroxine in the developmental hippocampus. J Neuroendocrinol 17(12):836–845
Schaefer S, Vogt T, Nowak T, Kann PH (2008) Pituitary function and the somatotrophic system in patients with idiopathic Parkinson's disease under chronic dopaminergic therapy. J Neuroendocrinol 20(1):104–109
Munhoz RP, Teive HA, Troiano AR, Hauck PCR, Leiva MHH, Graff H et al (2004) Parkinson's disease and thyroid dysfunction. Parkinsonism Relat Disord 10(6):381–383
Bonuccelli U, D'Avino C, Caraccio N, Del Guerra P, Casolaro A, Pavese N et al (1999) Thyroid function and autoimmunity in Parkinson's disease: a study of 101 patients. Parkinsonism Relat Disord 5(1–2):49–53
Vairetti M, Ferrigno A, Rizzo V, Ambrosi G, Bianchi A, Richelmi P et al (2012) Impaired hepatic function and central dopaminergic denervation in a rodent model of Parkinson's disease: a self-perpetuating crosstalk? Biochim Biophys Acta BBA Mol Basis Dis 1822(2):176–184
Aziz N, Pijl H, Frölich M, Roelfsema F, Roos R (2011) Diurnal secretion profiles of growth hormone, thyrotrophin and prolactin in Parkinson’s disease. J Neuroendocrinol 23(6):519–524
Umehara T, Matsuno H, Toyoda C, Oka H (2015) Thyroid hormone level is associated with motor symptoms in de novo Parkinson’s disease. J Neurol 262(7):1762–1768
Salama M, Helmy B, El-Gamal M, Reda A, Ellaithy A, Tantawy D et al (2013) Role of L-thyroxin in counteracting rotenone induced neurotoxicity in rats. Environ Toxicol Pharmacol 35(2):270–277
Lima FR, Gervais A, Colin C, Izembart M, Neto VM, Mallat M (2001) Regulation of microglial development: a novel role for thyroid hormone. J Neurosci 21(6):2028–2038
Kihara M, Kihara Y, Tukamoto T, Nishimura Y, Watanabe H, Hanakago R et al (1993) Assessment of sudomotor dysfunction in early Parkinson's disease. Eur Neurol 33(5):363–365
Goldstein D, Holmes C, Dendi R, Bruce S, Li S-T (2002) Orthostatic hypotension from sympathetic denervation in Parkinson’s disease. Neurology 58(8):1247–1255
Hotta H, Onda A, Suzuki H, Milliken P, Sridhar A (2017) Modulation of calcitonin, parathyroid hormone, and thyroid hormone secretion by electrical stimulation of sympathetic and parasympathetic nerves in anesthetized rats. Front Neurosci 11:375
Melander A, Ericson L, Sundler F, Ingbar S (1974) Sympathetic innervation of the mouse thyroid and its significance in thyroid hormone secretion. Endocrinology 94(4):959–966
Stolakis V, Kalafatakis K, Botis J, Zarros A, Liapi C (2010) The regulatory role of neurotensin on the hypothalamic–anterior pituitary axons: emphasis on the control of thyroid-related functions. Neuropeptides 44(1):1–7
St-Gelais F, Jomphe C, Trudeau L-É (2006) The role of neurotensin in central nervous system pathophysiology: what is the evidence? J Psychiatry Neurosci 31(4):229–245
Cacabelos R (2017) Parkinson's disease: from pathogenesis to pharmacogenomics. Int J Mol Sci 18(3):551
Berger JR, Kelley RE (1981) Thyroid function in Parkinson disease. Neurology 31(1):93–95
Krulich L (1982) Neurotransmitter control of thyrotropin secretion. Neuroendocrinology 35(2):139–147
Vergès B, Giroud M, Vaillant G, Verges-Patois B, Brun J, Putelat R (1992) Ultrasensitive TSH assay and anti-parkinsonian treatment with levodopa. J Neurol Neurosurg Psychiatry 55(12):1210
Pourmirbabaei S, Dolatshahi M, Rahmani F (2019) Pathophysiological clues to therapeutic applications of glutamate mGlu5 receptor antagonists in levodopa-induced dyskinesia. Eur J Pharmacol 855:149–159
Cusimano G, Capriani C, Bonifati V, Meco G (1991) Hypothalamo-pituitary function and dopamine dependence in untreated parkinsonian patients. Acta Neurol Scand 83(3):145–150
Polleri A, Carolei A, Rolandi E, Masturzo P, Meco G, Agnoli A (1977) Changes in pituitary hormones serum levels in bromocryptine-treated parkinsonian patients. Neuropsychobiology 3(1):42–48
Chen SF, Yang YC, Hsu CY, Shen YC (2020) Risk of Parkinson's disease in patients with hypothyroidism: a nationwide population-based cohort study. Parkinsonism Relat Disord 74:28–32
Fernández E, García-Moreno JM, de Pablos MA, Chacón J (2014) May the thyroid gland and thyroperoxidase participate in nitrosylation of serum proteins and sporadic Parkinson's disease? Antioxidants Redox Signal 21(15):2143–2148
Tandeter H, Levy A, Gutman G, Shvartzman P (2001) Subclinical thyroid disease in patients with Parkinson's disease. Arch Gerontol Geriatr 33(3):295–300
Munhoz RP, Teive HA, Troiano AR, Hauck PR, Herdoiza Leiva MH, Graff H et al (2004) Parkinson's disease and thyroid dysfunction. Parkinsonism Relat Disord 10(6):381–383
da Cunha ME, dos Santos PR, Goes TC, de Carvalho VCB, Teixeira-Silva F, Stevens HE et al (2019) Effects of a rat model of gestational hypothyroidism on forebrain dopaminergic, gabaergic, and serotonergic systems and related behaviors. Behav Brain Res 366:77–87
Chaker L, Bianco AC, Jonklaas J, Peeters RP (2017) Hypothyroidism. Lancet (Lond Engl) 390(10101):1550–1562
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR (2015) Oxidative stress and Parkinson's disease. Front Neuroanat 9:91
Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E et al (2016) Thyroid hormones, oxidative stress, and inflammation. Mediat Inflamm 2016:6757154
Chakrabarti SK, Ghosh S, Banerjee S, Mukherjee S, Chowdhury S (2016) Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab 20(5):674–678
Petrovic N, Cvijic G, Davidović V (2003) Thyroxine and tri-iodothyronine differently affect uncoupling protein-1 content and antioxidant enzyme activities in rat interscapular brown adipose tissue. J Endocrinol 176:31–38
Venditti P, Balestrieri M, Di Meo S, Leo T (1997) Effect of thyroid state on lipid peroxidation, antioxidant defences, and susceptibility to oxidative stress in rat tissues. J Endocrinol 155:151–157
Sanyal D, Raychaudhuri M (2016) Hypothyroidism and obesity: an intriguing link. Indian J Endocrinol Metab 20(4):554–557
Longhi S, Radetti G (2013) Thyroid function and obesity. J Clin Res Pediatr Endocrinol 5(Suppl 1):40–44
Martin-Jimenez CA, Gaitan-Vaca DM, Echeverria V, Gonzalez J, Barreto GE (2017) Relationship between obesity, Alzheimer's disease, and Parkinson's disease: an astrocentric view. Mol Neurobiol 54(9):7096–7115
Mohammadi S, Dolatshahi M, Zare-Shahabadi A, Rahmani F (2019) Untangling narcolepsy and diabetes: pathomechanisms with eyes on therapeutic options. Brain Res 1718:212–222
Mazon JN, de Mello AH, Ferreira GK, Rezin GT (2017) The impact of obesity on neurodegenerative diseases. Life Sci 182:22–28
Hage M, Zantout MS, Azar ST (2011) Thyroid disorders and diabetes mellitus. J Thyroid Res 2011:439463
Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J (2007) Type 2 diabetes and the risk of Parkinson's disease. Diabetes Care 30(4):842
Pagano G, Polychronis S, Wilson H, Giordano B, Ferrara N, Niccolini F et al (2018) Diabetes mellitus and Parkinson disease. Neurology 90(19):e1654–e1662
Galvagnion C (2017) The role of lipids interacting with alpha-synuclein in the pathogenesis of Parkinson's disease. J Parkinson's Dis 7(3):433–450
Doria M, Maugest L, Moreau T, Lizard G, Vejux A (2016) Contribution of cholesterol and oxysterols to the pathophysiology of Parkinson's disease. Free Radic Biol Med 101:393–400
Bykov K, Yoshida K, Weisskopf MG, Gagne JJ (2017) Confounding of the association between statins and Parkinson disease: systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 26(3):294–300
Rahmani F, Aarabi MH (2017) Does apolipoprotein A1 predict microstructural changes in subgenual cingulum in early Parkinson? J Neurol 264(4):684–693
Vitali C, Wellington CL, Calabresi L (2014) HDL and cholesterol handling in the brain. Cardiovasc Res 103(3):405–413
Krausz Y, Freedman N, Lester H, Newman JP, Barkai G, Bocher M et al (2004) Regional cerebral blood flow in patients with mild hypothyroidism. J Nucl Med 45(10):1712–1715
Utku U, Gokce M, Ozkaya M (2011) Changes in cerebral blood flow velocity in patients with hypothyroidism. Eur J Endocrinol 165(3):465–468
Iadecola C (2017) The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96(1):17–42
Hong CT, Huang YH, Liu HY, Chiou HY, Chan L, Chien LN (2016) Newly diagnosed anemia increases risk of Parkinson's disease: a population-based cohort study. Sci Rep 6:29651
Santiago JA, Bottero V, Potashkin JA (2017) Biological and clinical implications of comorbidities in Parkinson's disease. Front Aging Neurosci 9:394
Visanji NP, Collingwood JF, Finnegan ME, Tandon A, House E, Hazrati LN (2013) Iron deficiency in parkinsonism: region-specific iron dysregulation in Parkinson's disease and multiple system atrophy. J Parkinson's Dis 3(4):523–537
Mostile G, Cicero CE, Giuliano L, Zappia M, Nicoletti A (2017) Iron and Parkinson's disease: a systematic review and meta-analysis. Mol Med Rep 15(5):3383–3389
Defazio G, Esposito M, Abbruzzese G, Scaglione CL, Fabbrini G, Ferrazzano G et al (2017) The Italian Dystonia Registry: rationale, design and preliminary findings. Neurol Sci 38(5):819–825
Caradoc-Davies T (1986) Resolution of dyskinesia and the" on-off" phenomenon in thyrotoxic patients with Parkinson's disease after antithyroid treatment. Br Med J (Clin Res Ed) 293(6538):38
Kim HT, Edwards MJ, Narsimhan RL, Bhatia KP (2005) Hyperthyroidism exaggerating parkinsonian tremor: a clinical lesson. Parkinsonism Relat Disord 11(5):331–332
Davies J, Morrish P, Scanlon M (2001) Graves’ disease presenting as hemiparkinsonism. J Endocrinol Investig 24(3):188–189
Venditti P, Di Stefano L, Di Meo S (2013) Vitamin E management of oxidative damage-linked dysfunctions of hyperthyroid tissues. Cell Mol Life Sci 70(17):3125–3144
Baizabal-Carvallo JF, Jankovic J (2018) Autoimmune and paraneoplastic movement disorders: an update. J Neurol Sci 385:175–184
Zhou JY, Xu B, Lopes J, Blamoun J, Li L (2017) Hashimoto encephalopathy: literature review. Acta Neurol Scand 135(3):285–290
Dong YH, Fu DG (2014) Autoimmune thyroid disease: mechanism, genetics and current knowledge. Eur Rev Med Pharmacol Sci 18(23):3611–3618
Otsuka J, Hida A, Ogyu K, Minamimoto R, Takeuchi S (2017) Improved 123I-Ioflupane binding after immunotherapy in anti-NAE antibody-positive hashimoto encephalopathy that clinically mimicked multiple system atrophy. Clin Nucl Med 42(8):e390–e391
Barbas H, Saha S, Rempel-Clower N, Ghashghaei T (2003) Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci 4:25
Honorat JA, McKeon A (2017) Autoimmune movement disorders: a clinical and laboratory approach. Curr Neurol Neurosci Rep 17(1):4
Jankovic J (2018) Immunologic treatment of Parkinson's disease. Future Med
Miranda M, Bustamante ML, Campero M, Wainstein E, Toche P, Espay AJ et al (2018) Movement disorders in non-encephalopathic Hashimoto's thyroiditis. Parkinsonism Relat Disord 55:141–142
Fauzi NAM, Abdullah S, Tan AH, Ramli NM, Tan CY, Lim S-Y (2019) Relapsing encephalopathy with dancing eyes and jerky limbs. Parkinsonism Relat Disord
Waln O, Jankovic J (2015) Paroxysmal movement disorders. Neurol Clin 33(1):137–152
Rana AQ, Nadeem A, Yousuf MS, Kachhvi ZM (2013) Late onset of atypical paroxysmal non-kinesigenic dyskinesia with remote history of Graves' disease. J Neurosci Rural Pract 4(4):449–450
Delhasse S, Debove I, Arnold-Kunz G, Ghika JA, Chabwine JN (2019) Erratic movement disorders disclosing Graves' disease and paralleling thyroid function but not autoantibody levels. J Int Med Res
Jansson B, Jankovic J (1985) Low cancer rates among patients with Parkinson's disease. Ann Neurol 17(5):505–509
Peretz C, Gurel R, Rozani V, Gurevich T, El-Ad B, Tsamir J et al (2016) Cancer incidence among Parkinson's disease patients in a 10-yrs time-window around disease onset: a large-scale cohort study. Parkinsonism Relat Disord 28:68–72
Cook IJ (2009) Oropharyngeal dysphagia. Gastroenterol Clin N Am 38(3):411–431
Napolitano F, D'Angelo L, de Girolamo P, Avallone L, de Lange P, Usiello A (2018) The Thyroid-target gene Rhes, a novel crossroad for neurological and psychiatric disorders: new insights from animal models. Neuroscience
Haque M, Kendal JK, MacIsaac RM, Demetrick DJ (2016) WSB1: from homeostasis to hypoxia. J Biomed Sci 23(1):61
de Castro WJP, Fonseca TL, Ueta CB, McAninch EA, Abdalla S, Wittmann G et al (2015) Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Investig 125(2):769–781
Cuadrado A, Garcı́a-Fernández LF, Imai T, Okano H, Muñoz A, (2002) Regulation of tau RNA maturation by thyroid hormone is mediated by the neural RNA-binding protein musashi-1. Mol Cell Neurosci 20(2):198–210
Lambeth JD, Krause K-H, Clark RA (eds) (2008) NOX enzymes as novel targets for drug development. Seminars in immunopathology. Springer, New York
Bandmann O, Davis M, Mrasden C, Wood N (1996) The human homologue of the weaver mouse gene in familial and sporadic Parkinson's disease. Neuroscience 72(4):877–879
Blum M, Weickert C, Carrasco E (1999) The weaver GIRK2 mutation leads to decreased levels of serum thyroid hormone: characterization of the effect on midbrain dopaminergic neuron survival. Exp Neurol 160(2):413–424
Li J-Q, Tan L, Yu J-T (2014) The role of the LRRK2 gene in Parkinsonism. Mol Neurodegener 9(1):47
Looyenga BD, Furge KA, Dykema KJ, Koeman J, Swiatek PJ, Giordano TJ et al (2011) Chromosomal amplification of leucine-rich repeat kinase-2 (LRRK2) is required for oncogenic MET signaling in papillary renal and thyroid carcinomas. Proc Natl Acad Sci USA 108(4):1439–1444
Gatto EM, Parisi V, Converso DP, Poderoso JJ, Carreras MC, Martí-Massó JF et al (2013) The LRRK2 G2019S mutation in a series of Argentinean patients with Parkinson's disease: clinical and demographic characteristics. Neurosci Lett 537:1–5
Funding
This study did not receive any grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any study with human participants or animals performed by any of the authors.
Informed consent
For this study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadi, S., Dolatshahi, M. & Rahmani, F. Shedding light on thyroid hormone disorders and Parkinson disease pathology: mechanisms and risk factors. J Endocrinol Invest 44, 1–13 (2021). https://doi.org/10.1007/s40618-020-01314-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01314-5