Abstract
Digitized assessments have a considerable potential to guide clinicial decision making and monitor progress and disease trajectories. The Timed Up and Go test (TUG) has been long established for assessment in geriatric medicine and instrumented versions (iTUG) have been developed and validated. This scoping review includes studies that applied the iTUG and aims to identify use cases to show where and how iTUG assessment could guide interventions and clinical management. The literature search was limited to peer-reviewed studies that performed pre- and post-intervention measurements with a 3-meter TUG instrumented with body-worn technology in samples of at least 20 subjects aged 60+ years. Of 3018 identified articles 20 were included. Four clinical use cases were identified: stratification for subsequent therapy, monitoring of disease or treatment-associated changes and evaluation of interventions in patients with idiopathic normal pressure hydrocephalus (1), and patients with Parkinson’s disease (2); monitoring after joint replacement surgery (3), and evaluation after different exercise and rehabilitation interventions (4). The included studies show diversity in terms of iTUG technology and procedures. The identified use cases highlight clinical relevance and high potential for the clinical application of the iTUG. A consensual approach as well as comprehensive reporting would help to further exploit the potential of the iTUG to support clinical management. Future studies should investigate the benefits of segmental iTUG analysis, responsiveness and participants’ perspectives on clinically meaningful changes in iTUG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mobility is a key indicator of quality of life in older people [1]. The measurement of physical activity and capacity as important determinants of mobility has made tremendous progress over the last decade. While physical activity measurements (‘what DO they do?’) focus on actual performance in real life (e.g., daily step count) [2], physical capacity tests (‘what CAN they do?’) assess the ability to perform physical tasks [3]. It has been demonstrated that capacity testing can be used as a proxy of health status, to detect preclinical disability, to predict future outcomes, to identify groups of people at risk, and to monitor changes over time in individuals or groups of older people [3, 4]. Among the most commonly physical capacity assessments used in older populations are the Timed Up and Go test (TUG) [5], the Short Physical Performance Battery [6], Chair Rise maneuvers [7], the six-minute walk test [8] and gait speed measurements at habitual and fast pace.
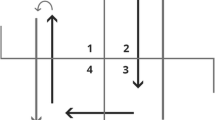
During the TUG, the test person stands up from a chair, walks 3 m at usual pace, turns around, walks 3 m back, and sits down on the chair. The time needed to complete the TUG is recorded by a trained assessor. The TUG test procedure can be considered as special among the most established physical capacity tests for older people, as it combines different routine movements (e.g., sit-to-stand, gait initiation) and multiple directions of movement (e.g., forward walking, 180 degree turning). The TUG is popular for its simplicity [9], as it requires minimal equipment and space. Since more than 30 years the TUG has been established as a clinical standard test to study mobility during the aging process, also in the context of many age-associated diseases and geriatric syndromes [5]. Due to its profoundly established validity and reliability the World Health Organization recommends the TUG for balance assessment in older people in clinical and research settings [10].
More recently, instrumented versions of the TUG (iTUG) have been developed and validated in older populations with and without specific pathologies. For example, one study showed that the iTUG using portable inertial sensors was able to detect specific gait deficits in patients with Parkinson’s disease (PwPD) and that the results of iTUG components were associated with disease severity [11]. The authors of another recent study found that a smartphone-based iTUG applied in community-dwelling older people could predict scores of the Community Balance and Mobility Scale (CBM), a valid, reliable and comprehensive performance-based assessment for measuring physical function in older people [12].
Previous reviews on the iTUG focused on the different technologies utilized for TUG instrumentation [13], feasibility of the iTUG in mobile devices [14], and technological proposals in studies on fall risk analysis [15]. Two major approaches have matured to objectively and accurately capture a subject’s gait pattern: wearable inertial measurement units (IMUs) and non-portable systems such as camera-based optical motion capturing [13]. Unlike stopwatch measurement, instrumented approaches allow the segmentation of the TUG, i.e., sit-to-stand, walk 1 (away from chair), turn 1 (around marker), walk 2 (back to chair), turn 2 (before sitting), and stand-to-sit movement [13].
There is consensus that benefits of the iTUG compared to the traditional stopwatch version include additional data on performance parameters, objective measurement, automated data collection and processing that can facilitate long-term analysis. In addition, the iTUG has the potential to be self-administered in the home environment of older people [13,14,15]. However, there are certain barriers to the implementation of instrumented tests, such as cost for buying medically certified products, and additional training of assessment staff [13]. In addition, kinematic parameters, such as angular velocity, are still unfamiliar to healthcare professionals.
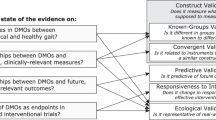
From the perspective of physiotherapists, geriatricians, other medical specialists, and allied health care professionals, there is a growing interest to understand how the iTUG could guide clinical management and interventions in older patients. The development and implementation of technologies as measurement tools in the clinical setting must also be compliant with the regulatory requirements for medical devices [16]. The Food and Drug Administration (FDA) and the National Institutes of Health (NIH) developed the “Biomarkers, EndpointS and other Tools” (BEST) recommendations [17] to implement, harmonise the terminology and advance the adoption in translational medicine and medical product development. It describes distinct use cases for digital biomarkers and clinical outcome assessments.
Our main research question was to identify studies that have used the total iTUG duration to examine differences before and after an intervention. Secondly, we were interested if the identified studies also used a segmental analysis of the TUG maneuver. Finally, we wanted to describe the applied technology and procedures (e.g., placement of the device, number of repetitions etc.). Our research interest and search strategy included the identification of other geriatric use cases for the iTUG to guide clinical decision making.
This scoping review is part of the SMART-AGE project (P2019-01-003 + https://smart-age.psychologie.uni-heidelberg.de/). Within this project, the iTUG is being used for process evaluation of the Keep On Keep Up App (KOKU). This app was developed by the University of Manchester [18]. Final results of the SMART-AGE project are expected in 2025.
Methods
Protocol and registration
This report adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) [19] (Supplement 1). The study protocol has been registered prior to the screening process (https://osf.io/exjz2/). A preliminary search in PubMed and PROSPERO was conducted and no current or ongoing reviews on the topic were identified.
Eligibility criteria
We were interested in identifying studies with different populations of older adults, including those with common age-associated diseases, evaluating surgical, pharmaceutical, training and other types of interventions by means of the iTUG. Studies needed to apply an instrumented version of the TUG in a sample with a mean age of 60 years and above. Studies needed to use wearable technologies for the iTUG assessment as only the latter provide an increased clinical utility when compared to more complex systems, i.e., ambient or camera systems that cannot be used in outpatient settings. Peer-reviewed studies published since 2012 were included as the technologies developed prior to the last ten years might not meet the current technological standards. Studies needed to measure the effect of an intervention over at least two timepoints. Due to the limited power of smaller pilot studies, trials were required to include at least 20 individuals in their analysis.
Studies were excluded based on populations experiencing congenital, non-age-related, or orphan diseases. Studies using the iTUG at baseline to predict outcomes such as falls without a follow-up measurement were not included. The articles had to comply with the standard version of the TUG using the 3 m distance [5]. Many home-dwellers have spatial restrictions not allowing gait assessments of more than 3 m. A full list of the inclusion and exclusion criteria can be found in Supplement 2.
Search and selection of sources of evidence
To identify potential articles, the electronic databases PubMed/MEDLINE, CINAHL, Web of Science, Cochrane Library and IEEE Xplore were searched on July 6, 2022. Reference lists of three relevant reviews on the iTUG [13,14,15] and of included articles were searched.
Search fields included title, abstracts, and Medical Subject Headings (MeSH). The search strategy used in PubMed/MEDLINE can be found in Supplement 3.
Titles and abstract screening was conducted by two reviewers (MJB, SL) independently using Rayyan (http://rayyan.qcri.org; [20]). Full-text screening was conducted independently by at least two reviewers (MJB, SL, KGO). Any disagreements were solved through discussion.
Data extraction process
Two reviewers (MJB, SL) filled out the data charting form independently. A third reviewer (KGO) was involved in case of conflict.
The following article characteristics were extracted: (a) general characteristics of the included studies (first author, year of publication, country in which study was conducted, population, sample size, sex, age, recruitment setting, study design, intervention description), (b) iTUG-related study characteristics (iTUG distance, technology, type of sensors, speed instruction, body placement of the technology used, number of iTUG repetitions, analysed segments, purpose of instrumentation), (c) pre-post differences within groups reported for iTUG total duration (pre- and post-intervention mean total iTUG duration) and pre-post differences within groups reported for iTUG segments sit-to-stand, walk 1, turn 1, walk 2, turn 2, stand-to-sit (improvement/worsening compared to baseline). In case of missing information, the articles’ corresponding authors were contacted via email or ResearchGate up to two times in intervals of two weeks.
Critical appraisal of individual sources of evidence
Methodological quality of the articles was assessed using the “before-after (pre-post) studies with no control group” Quality Assessment Tool of the United States National Institute of Health (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). The items were specified according to the purpose of this scoping review, e.g., the item on assessment was explicitly rated regarding iTUG assessment. Quality assessment was conducted independently by at least two reviewers (MJB, SL, KGO). In case of disagreement, the results were discussed until consensus was reached. A detailed description of the applied decision criteria are provided in Supplement 4.
Synthesis of results
Results are reported in clusters of diagnoses or interventions, mainly in form of tables. Along the screening process, we decided to split the reporting of results into two parts: first, we report the changes in total iTUG durations before and after intervention, followed by the changes in iTUG segments.
Results
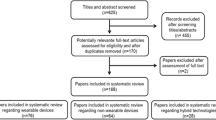
The search yielded 3018 records of which 312 underwent full-text review. Of these, N = 20 records were included. The PRISMA-ScR flow diagram [19] containing the number of identified and excluded articles at each stage of the search and selection process is displayed in Supplement 5.
The included studies were divided into five groups: Patients with idiopathic normal-pressure hydrocephalus (PwiNPH) [21,22,23,24], Patients with Parkinson’s disease (PwPD) [25, 26], elective orthopedic surgery [27, 28], exercise and rehabilitation interventions [29,30,31,32,33,34,35,36,37,38], and assistive devices [39, 40].
Study characteristics
The average sample size of the included studies was 43 (range: 20–119) with five (25%) studies including more than 50 individuals (Table 1). The mean age of the samples ranged from 60 to 91 years. Most of the studies (n = 11) were conducted in inpatient settings, i.e., hospital, clinic, or inpatient rehabilitation. Other settings were community-dwelling (n = 4), outpatient rehabilitation/clinic (n = 2), residential care/care home (n = 2), and day care center (n = 1). Ten studies included physical exercise interventions (e.g., Tai Chi), two applied drug therapies (i.e., l-dopa), five examined effects of surgical or invasive procedures (e.g., arthroplasty, cerebrospinal fluid tap-test) and two studies applied assistive devices (i.e., vibratory stimulation, orthosis). Depending on the type and duration of the respective intervention, participants were measured on the same day (e.g., with and without orthosis), within a couple of days (e.g., 72 h after cerebrospinal fluid tap-test), or weeks after the end of treatment (e.g., 12 weeks after intervention).
iTUG related characteristics
The instructions on walking speed ranged from comfortable/preferred/habitual speed to as fast as possible (Table 2). The number of repetitions per assessment varied from one repetition up to six, with most studies (n = 18) conducting two or more repetitions. The majority of the studies (n = 15) used stand-alone wearable inertial sensors, three studies used sensors embedded in mobile devices, i.e., an iPhone. Two studies used a combination of both, i.e., inertial sensors and iPod Touch. Most studies used accelerometers measuring acceleration (m/s2) and gyroscopes measuring angular velocities (deg/s) (n = 19). In eight studies, magnetometers measuring the direction of Earth’s magnetic field were used additionally. The sensors were attached most often to the lumbar spine (n = 14). Other locations were shin, thigh, shoe, or navel. Different methods were used for attachment (Table 2).
Pre-post intervention differences of total iTUG duration and segmentation
Pre-post changes of the total iTUG duration as well as of the different iTUG segments are shown in Tables 3 and 4, respectively. Data on further kinematic iTUG measures such as angular velocity and acceleration were reported in 13 studies [21, 22, 25, 26, 29,30,31,32,33,34,35,36, 38].
The included studies showed a large variety for baseline iTUG results ranging from 6.3 to 35.1 s. A commonly used threshold for the total TUG performance to indicate normal vs. below normal mobility was 12 s [41]. In summary, eleven studies (55%) found a statistically significant reduction of the total iTUG duration after the interventions. Follow-up data were not available for two studies [24, 35]. The total iTUG duration at baseline (iTUGB, Table 3) and statistically significant pre-post intervention changes of the total iTUG durations as well as for segment durations are shown below for each group of participants studied. Supplement 6 provides information on trends (non-significant changes).
Patients with idiopathic normal-pressure hydrocephalus (PwiNPH)
In the study of Ferrari et al. (2020) [21], participants with pure iNPH (p-iNPH, iTUGB = 17.0 s) and secondary NPH (s-NPH, iTUGB = 22.0 s) showed a shortening of the iTUG total duration 24 h after the lumbar tap-test. The changes were more pronounced after 72 h after the tap-test. It was however not reported whether these differences were statistically significant [21].
The other three studies [22,23,24] also applied the iTUG after a tap-test. Participants with a positive tap-test result (i.e., improvement of symptoms defined by ≥ 1 point on the iNPH grading scale [24]), subsequently underwent ventriculoperitoneal shunt surgery (VPS). Total iTUG duration at baseline was between 12.5 and 24.1 s [22,23,24]. A reduction of the iTUG total duration was observed in all three studies 24, 72 or 96 h after the tap-test. However, none reported interference-statistical values. A statistically significant reduction of the total iTUG duration was described by Ferrari et al. (2022) 6 months after shunt surgery [22].
One week after the VPS surgery significantly shorter durations were found by Ishikawa et al. [24] for both walking segments as well as turn2 and stand-to-sit compared to baseline. When comparing post-tap-test and post-shunt measurements the duration was significantly shorter for walk1 [24]. Patients in the study conducted by Ferrari et al. [22] showed a significant shortening for the sit-to-stand, total walk, and stand-to-sit segment duration 6 months after surgery.
Patients with Parkinson’s disease (PwPD)
Two studies compared ON and OFF states of L-dopa medication in PwPD with a baseline total iTUG duration of 20.2 s in the study by Dibilio et al. [25] and 8.2 s in the study by Miller Koop et al. [26] at OFF state. Both studies observed significantly shorter total iTUG completion times in the ON versus OFF state. The time needed for turn1 was significantly shorter in both studies. In the study conducted by Dibilio et al. [25], both walking segments as well as turn2 were also significantly shorter during the ON state.
Orthopedic conditions
Patients planned for elective total knee replacement were categorized as moderate (TUGB = 12.6 s) and low function (TUGB = 21.6 s) based on their total iTUG time before surgery in the study by Bloomfield et al. [27]. Only the moderate function group showed a statistically significant shorter total iTUG duration 2 weeks after surgery. The low function group showed a statistically significant shorter total iTUG time after 12 weeks.
Perelgut et al. [28] observed patients following total hip replacement using a collared (iTUGB = 13.0 s) or collarless (iTUGB = 13.4 s) femoral stem. Patients showed significantly shorter total iTUG durations 52 weeks after surgery in both groups [28].
Exercise and rehabilitation interventions in different settings and populations
Three studies examined the effect of different exercise interventions in PwPD. Flood et al. [29] measured one PwPD intervention group participating in Lee Silverman Voice Treatment (LSVT-BIG®) therapy and two control groups (healthy control, no therapy control group). Mollinedo-Cardalda et al. [30] compared Mat Pilates with calisthenics. Picardi et al. [31] examined the effects of inpatient rehabilitation consisting of physiotherapy and occupational therapy [31]. The groups observed in the first two studies [29, 30] had a baseline total iTUG duration < 12 s and participants in the study of Picardi et al. [31] had a baseline value > 12 s (iTUGB = 15.8 s). All studies found significantly shorter total iTUG durations in the PwPD intervention group following the intervention with persisting effects after 13 weeks in one study [29]. Three measures were shown to be responsive to rehabilitation (small to medium effect size): iTUG total duration, trunk angular velocity in the sit-to-stand and turning phases [31]. PwPD in the Mat Pilates group [30] showed significantly shorter times for both walking and turn2 segments after intervention. Four weeks after the end of the intervention, the significantly shorter duration for turn2 was maintained and a significantly longer duration was observed regarding sit-to-stand and walk2 [30].
Four studies examined pre-post iTUG results in outpatient settings. Smith et al. [32] measured participants of the 6-week “Better Bones” strength and balance exercise program (iTUGB = 6.3 s) who had significantly shorter total iTUG durations after the intervention [32].
A significant decrease was observed for the sit-to-stand, walk1, turn1 and total walk segment duration [32]. Celletti et al. [33] used the iTUG to test the efficacy of an intervention in patients with low back pain (iTUGB = 13.4 s). After ten sessions of the “Back School Therapy” intervention a significantly shorter time for the iTUG total performance was documented. The results also showed a significantly decreased sit-to-stand segment duration [33]. In the study from Doheny et al. [34] participants of a 4-week step exercise program (iTUGB = 9.2 s) showed significantly shorter durations for the turn2 segment, however not for the total iTUG duration or other segments [34]. The controlled study from Williams et al. [35] investigated effects of a Tai Chi intervention in comparison to a non-exercising group of people with mild to moderate dementia. Information on total iTUG duration changes was not provided.
Three studies were realized in inpatient and institution settings. Caronni et al. [36] examined patients with peripheral neuropathy of the lower limbs (iTUGB = 18.7 s). Study participants received 5 to 6 weeks of physiotherapy and occupational therapy. They showed a significantly shortened iTUG total time as well as decreased segment durations after the intervention [36]. Cancela Carral et al. (2017) [37] investigated people aged > 80 years participating in a 3-month study comparing aerobic (iTUGB = 30.3 s), muscle resistance (iTUGB = 29.1 s) or joint mobility (iTUGB = 30.3 s) exercise programs. None of the groups showed significant pre-post differences for the total iTUG duration [37]. However, participants in the aerobic and muscle resistance exercise groups showed significantly shorter times for both walking segments. A significant decrease in sit-to-stand duration was observed in the muscle resistance exercise group, while the joint mobility exercise group showed significantly longer sit-to-stand, turn1 and stand-to-sit durations [37]. Nonagenarians participating in the study from Cancela Carral et al. [38] who took part in a 12-week strength training program (iTUGB = 28.1 s) showed a a trend towards a reduction of total iTUG duration during the last week of the intervention [38]. Significantly longer total iTUG times were observed in the non-exercising control group (iTUGB = 35.1 s). The results showed a significantly longer turn1 duration as well as a trend towards a reduction of walk1 and walk2 times in the intervention group, although theses results were not significant (see Supplement 6). In the control group, participants showed significantly increased sit-to-stand and stand-to-sit durations [38].
Assistive devices
After applying calf vibration in two groups with different baseline levels for the iTUG (moderate function: iTUGB = 11.3 s; low function: iTUGB = 24.0 s), the results of the study from Toosizadeh et al. [40] showed trends towards longer iTUG total duration in the moderate function group and shorter iTUG total duration in the low function group [40] (see Supplement 6). Results on statistical significance were not reported for total iTUG duration. A significant decrease in duration regarding turn1 and turn and sit was observed in the low function group [40]. Participants of the study from Yalla et al. [39] wearing an ankle foot orthosis in combination with shoes (iTUGB = 13.8 s) did not show significant changes regarding the total iTUG duration compared to wearing shoes alone.
iTUG measurement properties reported in the included studies
Five studies reported good or excellent test–retest reliability of the iTUG [22,23,24, 26, 34]. One study [26] conducted a correlation analysis between the iTUG and the Movement Disorders Unified Parkinson’s Disease Rating Scale motor score (MDS-UPDRS-III), but it did not document a statistically significant correlation. Two studies [24, 32] found a strong correlation between manually measured TUG and iTUG durations. Measures of the iTUG’s responsiveness were reported by Picardi et al. [31] showing a small to medium improvement after the intervention. The correlation between iTUG and MiniBESTest was not significant [31].
Critical appraisal of included studies
Detailed results of the methodological quality assessment are shown in Supplement 4. In several studies, information on the iTUG measurement procedures were missing and needed to be requested: performance of practice trials before the iTUG measurement, use of walking aids, attachment of the sensors (i.e., body-fixed to the skin vs. body-worn over clothing), performance instructions (e.g., preferred speed or as fast as possible), measurement time points (e.g., how many days/weeks after the intervention) and analysis of the repeated measurements (e.g., mean, best-of all iTUG trials). Ninety-three percent of the authors contacted provided the missing information.
Discussion
This scoping review presents an overview on intervention studies applying iTUG measurements in groups of older people. Patients with idiopathic normal pressure hydrocephalus (PwiNPH), patients with Parkinson's disease (PwPD), patients with elective joint replacement and older people participating in exercise and rehabilitation interventions were identified as clinical use cases. The following discussion focuses on clinical iTUG applications that are ready for use or at least approaching this state. We focus on results that are not only statistically significant and detectable in terms of a minimal detectable change (MDC) but which are also relevant from a clinical perspective classfied by the concept of minimal important difference (MID). There is inconsistency in the literature regarding the terms MID, MCID, MIC, MCIC. For the purpose of this scoping review, the currently most widely acclaimed terminology MID [42] will be used reflecting the smallest difference in the TUG score that clinicians and patients perceive as relevant or meaningful and that thereby may contribute to decision making in therapy planning [43, 44].
Clinical use case 1: stratification, evaluation and monitoring of PwiNPH
The most mature use case was identified in PwiNPH. The diagnosis of iNPH is based on imaging, medical history and a positive tap-test. The response of a tap-test is defined as a ≥ 1 point improvement on the iNPH grading scale assessing the severity of gait disturbance, cognitive impairment and urinary incontinence [24]. The included studies used the iTUG to quantitatively assess gait before and after a tap-test. Three studies observed an iTUG improvement ranging from 2 to 8.7 s after the tap-test. MID/MDC TUG values in PwiNPH were reported to be 3.6 s [45] to 5 s [22].
The change in mobility performance are key to recommend and perform a VPS surgery. In case of a positive tap-test result, a VPS is recommended. The results of the included studies show a reduction of the TUG total duration in the tap-test-positive groups after tap-test and after a subsequent VPS surgery confirming the diagnosis. This indicates that the iTUG could serve as a digital mobility outcome to stratify patients regarding eligibility for subsequent VPS surgery and to evaluate surgery success. The iTUG could also be useful for monitoring patients to control the VPS function [22], and potentially adapt valve function if needed.
In PwiNPH the iTUG is used on a n = 1 basis, with each patient serving as his or her own control. This offers the possibility of individualised clinical management. The recommendation of the most recent guideline from Japan for an invasive treatment (i.e., VPS surgery) is based on MRI imaging, medical history and results of the iTUG. The iTUG can be performed objectively as well as repeated and reproduced if needed [46].
The results of the included studies do not show to what extent specific segments of the iTUG could be particularly relevant for this use case.
Clinical use case 2: stratification, evaluation and monitoring of PwPD
A common clinical challenge is the differentiation between neurodegenerative PD and vascular parkinsonism. Cerebrovascular lesions can cause manifestations similar to PD. However, most of these patients are older at onset and have more prominent symptoms of the lower extremities [47]. l-dopa therapy is often started without objective tests, although this treatment is not indicated in patients with mixed pathology and patients may experience side effects such as orthostatic symptoms or nausea [47]. In this review, two studies used the iTUG to measure mobility in OFF and ON stages of medication with each patient serving as his or her own control [25, 26]. The MDC is around 3.5 s [44]. A change of > 3.5 s was found in one of the included PwPD studies [25]. In the other study, the iTUG difference was smaller and may not have been subjectively noticeable because the participants did not show any physical capacity deficits at baseline, as shown by mean iTUG values of < 9 s. In this clinical use case the iTUG could be useful for stratification purposes, e.g., de-prescribing medication for patients with parkinsonism due to vascular disease or patients with atypical PD.
PwPD are amongst the most analyzed participants in studies using iTUG approaches [13,14,15], therefore it is not surprising that this patient group was identified as a clinical use case for the iTUG. However, mobility problems, e.g., small shuffling steps, usually worsen with disease severity and are not regarded as pre-clinical or early stage symptoms of PD [47]. Therefore, the iTUG has a potential for evaluating and monitoring of PwPD with moderate-to-severe stages of PD [13] (Hoehn and Yahr stages II, III and IV) but it may not be suitable to establish the diagnosis of PD.
The iTUG also allows the specific analysis of turning maneuvers in PwPD, which is of particular relevance in this population, especially when the disease progresses [48]. In the included studies, changes in the turn segments were more likely to show significant improvements with therapy than the other segments [25, 26, 30]. The total iTUG duration and turning segment durations should be compared with a clinical gold standard and with Patient-Reported Outcome Measures (PROMs) such as the MDS-UPDRS-II/III. The iTUG may therefore be used as secondary endpoint. Previous research showed excellent reliability of wearable inertial sensor-based iTUG measurements in PwPD [49] as well as feasibility and sensitivity to detect mobility deficits in patients with PD at home [50].
Another perspective on iTUG application is its use to analyse freezing symptoms of PwPD. One study that classified PwPD by their history of freezing of gait found that turn segments were longer in freezers who showed greater improvements with medication [25]. Consequently, observing changes in the turning segment of the iTUG might be a relevant criterion in addition to the total duration for monitoring clinically relevant changes in PwPD. However, for the analysis of freezing of gait, multisensory approaches would be necessary (e.g., additional sensors worn on shoes [21, 22]), which are more complex, cost-intensive, and thus have a more limited clinical applicability than unisensory approaches. Ideally, the iTUG assessment of PwPD should have a mandatory seven-day mobility measurement, as this approach will also capture fluctuating and rare events, e.g., freezing or falls [51].
Clinical use case 3: monitoring after joint replacement surgery
Another potential use case is the use of the iTUG to evaluate surgical procedures, e.g., by monitoring patients before and after elective joint replacement surgery. Typically, the mobility of patients deteriorates in the first 2–3 weeks after the operation before significant improvements in physical capacity are observed. This is also shown in the results of two studies [27, 28]. MDC scores for iTUG measurements for knee (2.3 s [52]) and hip arthroplasty (1.6 s [53]) are reported in studies following patients for 6 months. Participants in both studies showed statistically significant and detectable iTUG changes. Participants with low mobility impairment before knee surgery had greater improvements in iTUG total duration 12 weeks after surgery (4.9 s) compared to those with moderate mobility impairment preoperatively (0.9 s) [27].
The iTUG could qualify as a clinical endpoint while changes in physical activity, pain, and quality of life may serve as additional endpoints for these patient groups. The iTUG also has the potential to compare the results of home-based and inpatient rehabilitation [54] as the assessment could be performed in an unsupervised manner. The available data on the individual TUG segments in the included studies do not allow any additional conclusions.
Clinical use case 4: evaluation of exercise and rehabilitation interventions
Six out of ten studies who used the iTUG to evaluate exercise and rehabilitation interventions showed a statistically significant improvement of the total iTUG duration [29,30,31,32,33, 36] ranging from 0.5 to 3.6 s.
In the three studies with PwPD moderate to large effects were reported from participants of LSVT-BIG®, Mat Pilates and inpatient rehabilitation (physiotherapy/occupational therapy) ranging from 1.6 to 1.9 s. These results are in line with results of other studies reporting positive effects of LSVT-BIG® and physiotherapy on motor performance [55, 56]. In the inpatient rehabilitation study, iTUG measures were responsive to the interventions applied. It remains unclear whether the differences in iTUG total duration are perceived as meaningful, as to the best of our knowledge no MID is available for exercise or rehabilitation interventions in PwPD. Particularly in the two studies with short baseline iTUG times < 10 s [29, 30], more challenging assessments such as the Community Balance and Mobility Scale (CMB) [57]) might be more appropriate to identify relevant changes in physical capacity.
Outpatient programs showed statistically significant changes being 0.5 s for the “Better Bones” strength and balance training and 2.1 s for the back school rehabilitation. The MDC reported in the “Better Bones” study was 0.8 s [32]. A MID value of 0.8 to 1.4 was reported for the TUG in a study observing outpatients with hip osteoarthritis undergoing exercise therapy [58]. Although they constitute a diffent patient population it can be assumed that the back school participants experienced an MID in their iTUG performance. This is also supported by the fact that the participants in this study surpassed the normal vs. below normal mobility threshold of 12 s [41]. It seems unlikely that the 0.5 s iTUG change in the “Better Bones” study was perceived as a meaningful change, particularly as the participants started with fast iTUG baseline durations of 6.3 s. It is noticeable that even if the total duration did not change significantly after the stepping intervention, the time needed for turn 2 was significantly shorter.
Inpatient rehabilitation (physiotherapy/occupational therapy) participants with peripheral neuropathy showed statistically significant changes of 3.6 s in iTUG performance at the end of rehabilitation. Day care and care home exercise programs for older people > 80 years did not show statistically significant improvements regarding iTUG performance although numerically large differences from + 3.5 s (slower iTUG) to − 4.9 s (faster iTUG) were reported. Standard deviations were large in all of the three inpatient studies indicating a sample size problem. In the day care study [38], sit-to-stand and walking segments improved, but the total iTUG duration did not change significantly.
Since only one study examined the responsiveness of the iTUG [31], it remains largely unclear whether the interventions in the other exercise and rehabilitation studies were actually ineffective or whether the applied iTUG procedure was not able to detect a true change.
In the two studies applying assistive devices significance was not reported. Therefore a use case could not be identified.
Methodological limitations
Most of the included studies were pilot studies. Their relevance is therefore limited due to their small sample size. Because of insufficient reporting on iTUG methodology in the included studies, we see a urgent need for a consensus to standardize the iTUG measurement and its reporting. This would improve the interpretability of results. In Textbox 1, we suggest recommendations for minimum reporting in studies using iTUG approches.
Recommendations and future directions
The iTUG has a high potential to be implemented by clinicians. iTUG algorithms enable a standardized and reproducible measurement of the TUG. An instrumented measurement allows the assessor to concentrate on observing and safeguard the patient without being distracted, e.g. when handling a stopwatch. The use of smartphones extends the scope of applicability from analogue inpatient measurement to remote monitoring and self-assessment in outpatient and inhome settings. The latter will be needed for an inclusive medical approach when access to inpatient services will be increasingly limited.
The need for further validation studies adhering to the COSMIN terminology of measurement properties is once again emphasized by the WHO Locomotor Capacity Working Group [10].
A recent systematic review [59] of MIDs for different balance measures used with older people in research and clinical settings shows that MIDs are still pending for many health conditions. Future studies should aim to determine iTUG responsiveness and MID thereby highlighting the ability of the iTUG to detect clinically important change [10, 60].
In some use cases other or more challenging assessments (e.g., CBM, MiniBEST test) should be used as additional measures to perform a comprehensive assessment of physical capacity. This is particularly relevant for patients with a higher performance level where the iTUG might have ceiling effects. Multiple repetitions of the iTUG (e.g., three to five) during one assessment could be beneficial to eliminate the variance in performance between the runs [12].
For the time being, we consider the analysis of kinematic parameters as clinically exploratory. It is unlikely that clinical experts will be familiar with angular velocities and acceleration values in the foreseeable future.
From a global perspective, feasible and widely applicable assessments are required, especially for use in low- and middle-income countries [10]. The iTUG holds great potential in this regard, as the measurement can be carried out almost at any time and in any location where a chair, a 3 m space and, e.g., a smartphone is available. Data gathered by using the iTUG could be digitally and automatically stored, shared and analyzed over long distances, enabling continuous monitoring and, if indicated, rapid action planning.
Conclusion
This scoping review reveals first use cases for the application of an iTUG for PwiNPH and PwPD. The iTUG could also be used to monitor and evaluate joint replacement surgery as well as exercise and rehabilitation interventions. Methodological and reporting limitations of the included studies currently affect interpretability. Therefore a consensus is required to guarantee a more harmonized performance and reporting in future studies and in clinical practice.
Data availability
No datasets were generated or analysed during the current study.
References
La Grow S, Yeung P, Towers A et al (2013) The impact of mobility on quality of life among older persons. J Aging Health 25:723–736. https://doi.org/10.1177/0898264313490198
Bootsma-van der Wiel A, Gussekloo J, de Craen AJ et al (2001) Disability in the oldest old: “can do” or “do do”? J Am Geriatr Soc 49:909–914. https://doi.org/10.1046/j.1532-5415.2001.49181.x
Kuh D, Karunananthan S, Bergman H et al (2014) A life-course approach to healthy ageing: maintaining physical capability. Proc Nutr Soc 73:237–248. https://doi.org/10.1017/S0029665113003923
Lamb SE, Keene DJ (2017) Measuring physical capacity and performance in older people. Best Pract Res Clin Rheumatol 31:243–254. https://doi.org/10.1016/j.berh.2017.11.008
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142–148. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x
Guralnik JM, Simonsick EM, Ferrucci L et al (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49:M85-94. https://doi.org/10.1093/geronj/49.2.m85
Jones CJ, Rikli RE, Beam WC (1999) A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 70:113–119. https://doi.org/10.1080/02701367.1999.10608028
Guyatt GH, Sullivan MJ, Thompson PJ et al (1985) The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 132:919–923
Hellmers S, Izadpanah B, Dasenbrock L et al (2018) Towards an automated unsupervised mobility assessment for older people based on inertial TUG measurements. Sensors (Basel). https://doi.org/10.3390/s18103310
Honvo G, Sabico S, Veronese N et al (2023) Measures of attributes of locomotor capacity in older people: a systematic literature review following the COSMIN methodology. Age Ageing 52:iv44–iv66. https://doi.org/10.1093/ageing/afad139
Zampieri C, Salarian A, Carlson-Kuhta P et al (2010) The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease. J Neurol Neurosurg Psychiatry 81:171–176. https://doi.org/10.1136/jnnp.2009.173740
Bergquist R, Nerz C, Taraldsen K et al (2020) Predicting advanced balance ability and mobility with an instrumented timed up and go test. Sensors (Basel). https://doi.org/10.3390/s20174987
Sprint G, Cook DJ, Weeks DL (2015) Toward automating clinical assessments: a survey of the timed up and go. IEEE Rev Biomed Eng 8:64–77. https://doi.org/10.1109/RBME.2015.2390646
Ponciano V, Pires IM, Ribeiro FR et al (2020) Is the timed-up and go test feasible in mobile devices? A Systematic Review Electronics (Basel) 9:528. https://doi.org/10.3390/electronics9030528
Ortega-Bastidas P, Gómez B, Aqueveque P et al (2023) Instrumented timed up and go test (iTUG)-more than assessing time to predict falls: a systematic review. Sensors (Basel) 23:3426. https://doi.org/10.3390/s23073426
Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC
FDA-NIH Biomarker Working Group (2020) BEST (Biomarkers, EndpointS, and other Tools) Resource. US: Food and Drug ADministration, National Institutes of Health
Stanmore E (2021) Developing, testing, and implementing a falls prevention and healthy aging app (keep-on-keep-up) for older adults. Innov Aging 5:514. https://doi.org/10.1093/geroni/igab046.1990
Tricco AC, Lillie E, Zarin W et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169:467–473. https://doi.org/10.7326/M18-0850
Ouzzani M, Hammady H, Fedorowicz Z et al (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5:210. https://doi.org/10.1186/s13643-016-0384-4
Ferrari A, Milletti D, Giannini G et al (2020) The effects of cerebrospinal fluid tap-test on idiopathic normal pressure hydrocephalus: an inertial sensors based assessment. J Neuroeng Rehabil 17:7. https://doi.org/10.1186/s12984-019-0638-1
Ferrari A, Milletti D, Palumbo P et al (2022) Gait apraxia evaluation in normal pressure hydrocephalus using inertial sensors. Clinical correlates, ventriculoperitoneal shunt outcomes, and tap-test predictive capacity. Fluids Barriers CNS 19:51. https://doi.org/10.1186/s12987-022-00350-y
Yamada S, Aoyagi Y, Yamamoto K et al (2019) Quantitative evaluation of gait disturbance on an instrumented timed up-and-go test. Aging Dis 10:23–36. https://doi.org/10.14336/AD.2018.0426
Ishikawa M, Yamada S, Yamamoto K et al (2019) Gait analysis in a component timed-up-and-go test using a smartphone application. J Neurol Sci 398:45–49. https://doi.org/10.1016/j.jns.2019.01.023
Dibilio V, Nicoletti A, Mostile G et al (2017) Dopaminergic and non-dopaminergic gait components assessed by instrumented timed up and go test in Parkinson’s disease. J Neural Transm (Vienna) 124:1539–1546. https://doi.org/10.1007/s00702-017-1794-8
Miller Koop M, Ozinga SJ, Rosenfeldt AB et al (2018) Quantifying turning behavior and gait in Parkinson’s disease using mobile technology. IBRO Rep 5:10–16. https://doi.org/10.1016/j.ibror.2018.06.002
Bloomfield RA, Williams HA, Broberg JS et al (2019) Machine learning groups patients by early functional improvement likelihood based on wearable sensor instrumented preoperative timed-up-and-go tests. J Arthroplasty 34:2267–2271. https://doi.org/10.1016/j.arth.2019.05.061
Perelgut ME, Polus JS, Lanting BA et al (2020) The effect of femoral stem collar on implant migration and clinical outcomes following direct anterior approach total hip arthroplasty. Bone Joint J 102-B:1654–1661. https://doi.org/10.1302/0301-620X.102B12.BJJ-2019-1428.R1
Flood MW, O’Callaghan BPF, Diamond P et al (2020) Quantitative clinical assessment of motor function during and following LSVT-BIG® therapy. J Neuroeng Rehabil 17:92. https://doi.org/10.1186/s12984-020-00729-8
Mollinedo-Cardalda I, Cancela-Carral JM, Vila-Suárez MH (2018) Effect of a mat pilates program with theraband on dynamic balance in patients with parkinson’s disease: feasibility study and randomized controlled trial. Rejuvenation Res 21:423–430. https://doi.org/10.1089/rej.2017.2007
Picardi M, Redaelli V, Antoniotti P et al (2020) Turning and sit-to-walk measures from the instrumented Timed Up and Go test return valid and responsive measures of dynamic balance in Parkinson’s disease. Clin Biomech (Bristol, Avon) 80:105177. https://doi.org/10.1016/j.clinbiomech.2020.105177
Smith E, Cunningham C, Greene BR et al (2021) Detecting subtle mobility changes among older adults: the Quantitative Timed Up and Go test. Aging Clin Exp Res 33:2157–2164. https://doi.org/10.1007/s40520-020-01733-7
Celletti C, Mollica R, Ferrario C et al (2020) Functional evaluation using inertial measurement of back school therapy in lower back pain. Sensors (Basel). https://doi.org/10.3390/s20020531
Doheny EP, McGrath D, Ditroilo M et al (2013) Effects of a low-volume, vigorous intensity step exercise program on functional mobility in middle-aged adults. Ann Biomed Eng 41:1748–1757. https://doi.org/10.1007/s10439-013-0804-8
Williams J, Nyman S (2021) A secondary analysis of a randomised controlled trial to investigate the effect of Tai Chi on the instrumented timed up and go test in people with mild to moderate dementia. Aging Clin Exp Res 33:2175–2181. https://doi.org/10.1007/s40520-020-01741-7
Caronni A, Picardi M, Pintavalle G et al (2019) Responsiveness to rehabilitation of balance and gait impairment in elderly with peripheral neuropathy. J Biomech 94:31–38. https://doi.org/10.1016/j.jbiomech.2019.07.007
Cancela Carral JM, Pallin E, Orbegozo A et al (2017) Effects of three different chair-based exercise programs on people older than 80 years. Rejuvenation Res 20:411–419. https://doi.org/10.1089/rej.2017.1924
Carral C, Cancela JM, Rodríguez AL et al (2019) Muscle strength training program in nonagenarians - a randomized controlled trial. Rev Assoc Med Bras 1992:851–856. https://doi.org/10.1590/1806-9282.65.6.851
Yalla SV, Crews RT, Fleischer AE et al (2014) An immediate effect of custom-made ankle foot orthoses on postural stability in older adults. Clin Biomech (Bristol, Avon) 29:1081–1088. https://doi.org/10.1016/j.clinbiomech.2014.10.007
Toosizadeh N, Wahlert G, Fain M et al (2020) The effect of vibratory stimulation on the timed-up-and-go mobility test: a pilot study for sensory-related fall risk assessment. Physiol Res 69:721–730. https://doi.org/10.33549/physiolres.934451
Bischoff HA, Stähelin HB, Monsch AU et al (2003) Identifying a cut-off point for normal mobility: a comparison of the timed “up and go” test in community-dwelling and institutionalised elderly women. Age Ageing 32:315–320. https://doi.org/10.1093/ageing/32.3.315
Dettori JR, Norvell DC, Chapman JR (2022) Clinically important difference: 4 tips toward a better understanding. Glob Spine J 12:1297–1298. https://doi.org/10.1177/21925682221092721
Schünemann HJ, Guyatt GH (2005) Commentary–goodbye M(C)ID! Hello MID, where do you come from? Health Serv Res 40:593–597. https://doi.org/10.1111/j.1475-6773.2005.00374.x
Huang S-L, Hsieh C-L, Wu R-M et al (2011) Minimal detectable change of the timed “up & go” test and the dynamic gait index in people with Parkinson disease. Phys Ther 91:114–121. https://doi.org/10.2522/ptj.20090126
Gallagher R, Marquez J, Osmotherly P (2019) Clinimetric properties and minimal clinically important differences for a battery of gait, balance, and cognitive examinations for the tap test in idiopathic normal pressure hydrocephalus. Neurosurgery 84:E378–E384. https://doi.org/10.1093/neuros/nyy286
Nakajima M, Yamada S, Miyajima M et al (2021) Guidelines for management of idiopathic normal pressure hydrocephalus (third edition): endorsed by the Japanese society of normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 61:63–97. https://doi.org/10.2176/nmc.st.2020-0292
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. The Lancet 397:2284–2303. https://doi.org/10.1016/S0140-6736(21)00218-X
Bertoli M, Della Croce U, Cereatti A et al (2019) Objective measures to investigate turning impairments and freezing of gait in people with Parkinson’s disease. Gait Posture 74:187–193. https://doi.org/10.1016/j.gaitpost.2019.09.001
Kleiner AFR, Pacifici I, Vagnini A et al (2018) Timed Up and Go evaluation with wearable devices: validation in Parkinson’s disease. J Bodyw Mov Ther 22:390–395. https://doi.org/10.1016/j.jbmt.2017.07.006
Zampieri C, Salarian A, Carlson-Kuhta P et al (2011) Assessing mobility at home in people with early Parkinson’s disease using an instrumented Timed Up and Go test. Parkinsonism Relat Disord 17:277–280. https://doi.org/10.1016/j.parkreldis.2010.08.001
Warmerdam E, Hausdorff JM, Atrsaei A et al (2020) Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol 19:462–470. https://doi.org/10.1016/S1474-4422(19)30397-7
Yuksel E, Kalkan S, Cekmece S et al (2017) Assessing minimal detectable changes and test-retest reliability of the timed up and go test and the 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty 32:426–430. https://doi.org/10.1016/j.arth.2016.07.031
Yuksel E, Unver B, Kalkan S et al (2021) Reliability and minimal detectable change of the 2-minute walk test and Timed Up and Go test in patients with total hip arthroplasty. Hip Int J Clin Exp Res Hip Pathol Ther 31:43–49. https://doi.org/10.1177/1120700019888614
Buhagiar MA, Naylor JM, Harris IA et al (2017) Effect of inpatient rehabilitation vs a monitored home-based program on mobility in patients with total knee arthroplasty: the HIHO randomized clinical trial. JAMA 317:1037–1046. https://doi.org/10.1001/jama.2017.1224
Ebersbach G, Ebersbach A, Edler D et al (2010) Comparing exercise in Parkinson’s disease—the Berlin LSVT®BIG study. Mov Disord 25:1902–1908. https://doi.org/10.1002/mds.23212
Schaible F, Maier F, Buchwitz TM et al (2021) Effects of Lee Silverman Voice Treatment BIG and conventional physiotherapy on non-motor and motor symptoms in Parkinson’s disease: a randomized controlled study comparing three exercise models. Ther Adv Neurol Disord 14:1756286420986744. https://doi.org/10.1177/1756286420986744
Gordt K, Mikolaizak AS, Taraldsen K et al (2020) Creating and validating a shortened version of the community balance and mobility scale for application in people who are 61 to 70 years of age. Phys Ther 100:180–191. https://doi.org/10.1093/ptj/pzz132
Wright AA, Cook CE, Baxter GD et al (2011) A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther 41:319–327. https://doi.org/10.2519/jospt.2011.3515
Low DC, Walsh GS (2022) The minimal important change for measures of balance and postural control in older adults: a systematic review. Age Ageing. https://doi.org/10.1093/ageing/afac284
Mokkink LB, Terwee CB, Patrick DL et al (2010) The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 19:539–549. https://doi.org/10.1007/s11136-010-9606-8
Acknowledgements
We would like to thank Ronny Bergquist, Christian Werner, Jochen Klenk, Volker Braun, Sabato Mellone and Tobias Braun for their support in the conceptual and content planning of the review.
Funding
Open Access funding enabled and organized by Projekt DEAL. This review is part of the 5-year project”Smart Aging in Community Contexts”: Testing Intelligent Assistive Systems for Self-regulation and Co-regulation under Real-Life Conditions “(SMART-AGE, P2019-01-003) at the University of Heidelberg which is financially supported by the Carl Zeiss Foundation.
Author information
Authors and Affiliations
Contributions
MJB, SL, CPJ, CB and KGO were involved in the study conception and design. An experienced librarian (VB) performed a systematic search of the literature. MJB, SL and KGO screened potential articles and evaluated the methodological quality of studies. MJB, SL, KGO and MS acquired data for analysis. MJB, KGO and CB performed interpretation of data. MJB and SL drafted the paper. KGO, CB, SL, CPJ, DS, EL, and JMB critically revised the draft manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there was no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Böttinger, M.J., Labudek, S., Schoene, D. et al. “TiC-TUG”: technology in clinical practice using the instrumented timed up and go test—a scoping review. Aging Clin Exp Res 36, 100 (2024). https://doi.org/10.1007/s40520-024-02733-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40520-024-02733-7