Abstract
Background
The aging heart is characterized by cellular and molecular changes leading to a decline in physiologic function and cardiac remodeling, specifically the development of myocyte hypertrophy and fibrosis. Transient receptor potential vanilloid 2 (TRPV2), a stretch-mediated channel and regulator of calcium homeostasis, plays a key role in the function and structure of the heart. TRPV2 also plays an important role in the adaptive and maladaptive compensatory mechanisms of the heart in response to pathologic and exercise-induced stress. Our current study seeks to elucidate the potential role of TRPV2 channels in the regulation of cardiac function in aging.
Methods
Wild-type (WT) and TRPV2 functional knockout (FKO) mice were aged out to various time points, and their cardiac function was measured using advanced echocardiography. Furthermore, we histologically analyzed the heart morphology to determine myocyte hypertrophy, the development of fibrosis and the relative expression of TRPV2.
Results
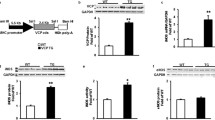
Our results demonstrate that even though TRPV2-FKO mice have impaired function at baseline, their cardiac function as measured via standard and advanced echocardiographic parameters (ejection fraction, cardiac output and circumferential strain) decreased less with aging in comparison with the WT group. Furthermore, there was less fibrosis and hypertrophy in the TRPV2-FKO group with aging in comparison with the WT. The expression of TRPV2 in the WT group did not significantly change with aging.
Conclusions
TRPV2 functional deletion is compatible with aging and associated with a decreased development of myocyte hypertrophy and fibrosis. It may be an important target for prevention of age-induced cardiac remodeling.







Similar content being viewed by others
References
Biernacka A, Frangogiannis NG (2011) Aging and cardiac fibrosis. Aging Dis 2:158–173
Dai D-F, Rabinovitch PS (2009) Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med 19:213–220. doi:10.1016/j.tcm.2009.12.004
Lakatta EG, Levy D (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation 107:346–354
North BJ, Sinclair DA (2012) The intersection between aging and cardiovascular disease. Circ Res 110:1097–1108. doi:10.1161/CIRCRESAHA.111.246876
Koch SE, Haworth KJ, Robbins N et al (2013) Age- and gender-related changes in ventricular performance in wild-type FVB/N mice as evaluated by conventional and vector velocity echocardiography imaging: a retrospective study. Ultrasound Med Biol 39:2034–2043. doi:10.1016/j.ultrasmedbio.2013.04.002
Muraki K, Iwata Y, Katanosaka Y et al (2003) TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93:829–838. doi:10.1161/01.RES.0000097263.10220.0C
Barro-Soria R, Stindl J, Müller C et al (2012) Angiotensin-2-mediated Ca2+ signaling in the retinal pigment epithelium: role of angiotensin-receptor-associated-protein and TRPV2 channel. PLoS ONE 7:e49624. doi:10.1371/journal.pone.0049624
Rubinstein J, Lasko VM, Koch SE et al (2014) Novel role of transient receptor potential vanilloid 2 in the regulation of cardiac performance. Am J Physiol Heart Circ Physiol 306:H574–H584. doi:10.1152/ajpheart.00854.2013
Iwata Y, Ohtake H, Suzuki O et al (2013) Blockade of sarcolemmal TRPV2 accumulation inhibits progression of dilated cardiomyopathy. Cardiovasc Res 99:760–768. doi:10.1093/cvr/cvt163
Iwata Y, Katanosaka Y, Arai Y et al (2009) Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Genet 18:824–834. doi:10.1093/hmg/ddn408
Naticchioni M, Karani R, Smith MA et al (2015) Transient receptor potential vanilloid 2 regulates myocardial response to exercise. PLoS ONE 10:e0136901. doi:10.1371/journal.pone.0136901
Guo Y, Flaherty MP, Wu W-J et al (2012) Genetic background, gender, age, body temperature, and arterial blood pH have a major impact on myocardial infarct size in the mouse and need to be carefully measured and/or taken into account: results of a comprehensive analysis of determinants of infarc. Basic Res Cardiol 107:288. doi:10.1007/s00395-012-0288-y
Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies (2011) Guide for the care and use of laboratory animals, 8th edn. The National Academies Press, Washington D.C., p 118
Park U, Vastani N, Guan Y et al (2011) TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci 31:11425–11436. doi:10.1523/JNEUROSCI.1384-09.2011
Koch SE, Gao X, Haar L et al (2012) Probenecid: novel use as a non-injurious positive inotrope acting via cardiac TRPV2 stimulation. J Mol Cell Cardiol 53:134–144. doi:10.1016/j.yjmcc.2012.04.011
Bauer M, Cheng S, Jain M et al (2011) Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res 108:908–916. doi:10.1161/CIRCRESAHA.110.239574
Katanosaka Y, Iwasaki K, Ujihara Y et al (2014) TRPV2 is critical for the maintenance of cardiac structure and function in mice. Nat Commun 5:3932. doi:10.1038/ncomms4932
Takahashi K, Fukushima S, Yamahara K et al (2008) Modulated inflammation by injection of high-mobility group box 1 recovers post-infarction chronically failing heart. Circulation. doi:10.1161/CIRCULATIONAHA.107.757443
Bauman TM, Nicholson TM, Abler LL et al (2014) Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. PLoS ONE 9:e109102. doi:10.1371/journal.pone.0109102
Bolte C, Zhang Y, Wang I-C et al (2011) Expression of Foxm1 transcription factor in cardiomyocytes is required for myocardial development. PLoS ONE 6:e22217. doi:10.1371/journal.pone.0022217
Ren X, Wang Y, Jones WK (2004) TNF-α is required for late ischemic preconditioning but not for remote preconditioning of trauma. J Surg Res 121:120–129. doi:10.1016/j.jss.2004.03.010
Ram R, Mickelsen DM, Theodoropoulos C et al (2011) New approaches in small animal echocardiography: imaging the sounds of silence. Am J Physiol Heart Circ Physiol 301:H1765–H1780. doi:10.1152/ajpheart.00559.2011
Azam S, Desjardins CL, Schluchter M et al (2012) Comparison of velocity vector imaging echocardiography with magnetic resonance imaging in mouse models of cardiomyopathy. Circ Cardiovasc Imaging 5:776–781. doi:10.1161/CIRCIMAGING.111.972406
Stein M, Boulaksil M, Jansen JA et al (2010) Reduction of fibrosis-related arrhythmias by chronic renin-angiotensin-aldosterone system inhibitors in an aged mouse model. Am J Physiol Heart Circ Physiol 299:H310–H321. doi:10.1152/ajpheart.01137.2009
Kass DA, Bronzwaer JGF, Paulus WJ (2004) What mechanisms underlie diastolic dysfunction in heart failure? Circ Res 94:1533–1542. doi:10.1161/01.RES.0000129254.25507.d6
Stypmann J, Engelen MA, Epping C et al (2006) Age and gender related reference values for transthoracic Doppler-echocardiography in the anesthetized CD1 mouse. Int J Cardiovasc Imaging 22:353–362. doi:10.1007/s10554-005-9052-9
Sandstede J, Lipke C, Beer M et al (2000) Age- and gender-specific differences in left and right ventricular cardiac function and mass determined by cine magnetic resonance imaging. Eur Radiol 10:438–442. doi:10.1007/s003300050072
Gardin JM, Henry WL, Savage DD et al (1979) Echocardiographic measurements in normal subjects: evaluation of an adult population without clinically apparent heart disease. J Clin Ultrasound 7:439–447. doi:10.1002/jcu.1870070606
Shibasaki K, Murayama N, Ono K et al (2010) TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J Neurosci 30:4601–4612. doi:10.1523/JNEUROSCI.5830-09.2010
Acknowledgements
We thank Drs. Michael Tranter and A. Phillip Owens III for thoughtful discussions and scientific input. We gratefully acknowledge Michael Caterina, Ph.D., for providing the initial breeding pairs of TRPV2-FKO mice.
Funding
Research reported in this publication was supported in part by the American Heart Association (Beginning Grant in Aid 13BGIA17140069 to JR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Rubinstein is a founding member of TRP Therapeutics. He has not received any fees or honoraria from this venture.
Human and animal rights
All animal procedures were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the University of Cincinnati (Cincinnati, Ohio, USA).
Informed consent
For this type of study formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jones, S., Mann, A., Worley, M.C. et al. The role of transient receptor potential vanilloid 2 channel in cardiac aging. Aging Clin Exp Res 29, 863–873 (2017). https://doi.org/10.1007/s40520-016-0663-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-016-0663-x