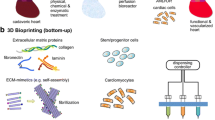
Abstract
Purpose of review
Although technological advancements in the genetic modification of pigs have renewed the interest in experiments involving the transplantation of whole pig organs into humans, complete human organs have not yet been fabricated. Organ fabrication, which involves the artificial creation of human organs, was explained based on the author’s latest research results. This study aimed to introduce the methods in the artificial creation of human organs that are currently undergoing research after showing the advantages and issues of the following three methods.
Recent findings
The first is a method of transplanting organoids that were created by culturing the remaining organs of a patient on a scaffold in vitro. The second is a method of injecting human progenitor cells into a scaffold of fetal organs from another species (e.g., pig) to make a chimeric organ. The third is a method of using machine perfusion to culture and to repair organs that are incompatible with transplantation.
Summary
Preclinical studies in pigs are advancing for all three methods, and the issues regarding clinical application are being overcome.

Similar content being viewed by others
Data and/or Code availability
All relevant data supporting the findings of this study are either included within the article or are available upon request from the corresponding author.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Porrett PM, Orandi BJ, Kumar V, Houp J, Anderson D, Cozette Killian A, et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am J Transplant. 2022;22:1037–53. https://doi.org/10.1111/ajt.16930.
Montgomery RA, Stern JM, Lonze BE, Tatapudi VS, Mangiola M, Wu M, et al. Results of two cases of pig-to-human kidney xenotransplantation. N Engl J Med. 2022;386:1889–98. https://doi.org/10.1056/NEJMoa2120238.
• Reardon S. First pig-to-human heart transplant: what can scientists learn? Nature. 2022;601:305–6. https://doi.org/10.1038/d41586-022-00111-9*(Article that describes the current barriers to xenotransplantation).
•• Kobayashi E, Tohyama S, Fukuda K. Organ fabrication using pigs as an in vivo bioreactor. Keio J Med. 2020;69:30–6. https://doi.org/10.2302/kjm.2019-0006-OA. **(Article by Eiji Kobayashi, the author of this paper, which summarizes the concept of creating human organs in the bodies of living animals).
Yamaguchi T, Sato H, Kato-Itoh M, Goto T, Hara H, Sanbo M, et al. Interspecies organogenesis generates autologous functional islets. Nature. 2017;542:191–6. https://doi.org/10.1038/nature21070.
Inui A, Sekine H, Sano K, Dobashi I, Yoshida A, Matsuura K, et al. Generation of a large-scale vascular bed for the in vitro creation of three-dimensional cardiac tissue. Regen Ther. 2019;11:316–23. https://doi.org/10.1016/j.reth.2019.10.001.
•• Sugimoto S, Kobayashi E, Fujii M, Ohta Y, Arai K, Matano M, et al. An organoid-based organ-repurposing approach to treat short bowel syndrome. Nature. 2021;592:99–104. https://doi.org/10.1038/s41586-021-03247-2. (**Article that is key to the Concept 1 as described in Table 1.)
Meran L, Massie I, Campinoti S, Weston AE, Gaifulina R, Tullie L, et al. Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure. Nat Med. 2020;26:1593–601. https://doi.org/10.1038/s41591-020-1024-z.
Gentilini MV, Rumbo M, Gondolesi GE. Organoid transplantation: new avenues to treat short bowel syndrome. Transplantation. 2021;105:2130–1. https://doi.org/10.1097/TP.0000000000003833.
• Kawai Y, Tohyama S, Arai K, Tamura T, Soma Y, Fukuda K, et al. Scaffold-free tubular engineered heart tissue from human induced pluripotent stem cells using Bio-3D printing technology in vivo. Front Cardiovasc Med. 2022;8: 806215. https://doi.org/10.3389/fcvm.2021.806215. (*Article that summarizes the unique research findings of assembling tissues in 3D.)
Saito Y, Matsumoto N, Yamanaka S, Yokoo T, Kobayashi E. Beneficial impact of interspecies chimeric renal organoids against a xenogeneic immune response. Front Immunol. 2022;13: 848433. https://doi.org/10.3389/fimmu.2022.848433.
•• Yokoo T, Yamanaka S, Kobayashi E. Xeno-regenerative medicine: A novel concept for donor kidney fabrication. Xenotransplantation. 2020;27: e12622. https://doi.org/10.1111/xen.12622. (**Article that reviews the Concept 2 as described in Table 1).
Takamura T, Matsui K, Matsumoto N, Saito Y, Fujimoto T, Tajiri S, et al. In vivo development of fetal pig kidneys in mature monkeys under clinically approved immunosuppressant drugs. Engineering. 2022;10:65–73. https://doi.org/10.1016/j.eng.2022.02.001.
•• Saito Y, Yamanaka S, Matsumoto N, Takamura T, Fujimoto T, Matsui K, et al. Generation of functional chimeric kidney containing exogenous progenitor-derived stroma and nephron via a conditional empty niche. Cell Rep. 2022;39: 110933. https://doi.org/10.1016/j.celrep.2022.110933. (**Article that describes the most recent research data on the Concept 2.)
Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557:50–6. https://doi.org/10.1038/s41586-018-0047-9.
Eshmuminov D, Becker D, Bautista Borrego L, Hefti M, Schuler MJ, Hagedorn C, et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat Biotechnol. 2020;38:189–98. https://doi.org/10.1038/s41587-019-0374-x.
• Clavien PA, Dutkowski P, Mueller M, Eshmuminov D, Bautista Borrego L, Weber A, et al. Transplantation of a human liver following 3 days of ex situ normothermic preservation. Nat Biotechnol. 2022 May 31. https://doi.org/10.1038/s41587-022-01354-7. (*Key article that describes the successful culture and transplantation of a human liver.)
Sampaziotis F, Muraro D, Tysoe OC, Sawiak S, Beach TE, Godfrey EM, et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371:839–46. https://doi.org/10.1126/science.aaz6964.
Yanagi Y, Nakayama K, Taguchi T, Enosawa S, Tamura T, Yoshimaru K, et al. In vivo and ex vivo methods of growing a liver bud through tissue connection. Sci Rep. 2017;7:14085. https://doi.org/10.1038/s41598-017-14542-2.
• Yoshimoto S, Ohara M, Torai S, Kasamatsu H, Ishikawa J, Kimura T, et al. Rapid metabolic recovery of donor circulatory death liver graft using whole blood perfusion: a pig study. Transplant Direct. 2021;7: e712. https://doi.org/10.1097/TXD.0000000000001170. (*Article that describes how an organ that had undergone circulatory arrest was revived.)
Acknowledgements
Sumitomo Pharma Co., Ltd., Japan (grant number: none, recipient: E.K.) and SCREEN Holdings Co., Ltd., Japan (grant number: none, recipient: E.K.). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of manuscript.
Funding
Sumitomo Pharma Co.,Ltd,SCREEN Holdings Co.,Ltd
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Eiji Kobayashi is a medical advisor of Sumitomo Pharma Co., Ltd., Japan and SCREEN Holdings Co., Ltd., Japan.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the author have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cellular Transplants
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobayashi, E. Organ Fabrication: Progress and Hurdles to Overcome. Curr Transpl Rep 9, 297–301 (2022). https://doi.org/10.1007/s40472-022-00372-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-022-00372-3