Abstract
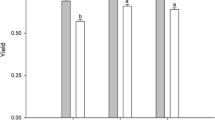
Studies on the effects of inorganic nutrients manipulation (specially nitrogen and phosphorus) in freshwater macroalgae are scarce. Physiological responses (growth, photosynthesis, and pigment contents) to nitrogen and phosphorus impoverishment (nitrate and phosphates, respectively) were analyzed under culture conditions in two populations of Compsopogon caeruleus coming from environments with distinct levels of saprobity (oligosaprobic and mesosaprobic, designated isolates o and m, respectively). The aim was to evaluate the isolate responses (decrease or increase in physiological performance) to decrease in inorganic nitrogen and phosphorus concentrations. Three dilutions in original concentration of nitrogen and phosphorus of Bold Basic Medium were tested. For the nitrogen treatments, the isolate m had a more pronounced decrease in general performance in comparison to isolate o: lower values of effective quantum yield and phycobiliprotein concentrations in all nitrogen dilutions. Phycobiliprotein degradation is a typical and widely reported response of red algae under nitrogen scarcity. For the phosphorus experiments, the isolate o showed a more pronounced decrease in general performance in comparison to isolate m: lower values of maximum photosynthetic rate (Pmax) and photosynthetic efficiency (α), besides lower phycobiliprotein concentrations in all dilutions. The best performance of C. caeruleus was found at higher nutrient concentrations, confirming previous records as a good bioindicator of enriched environments. Nevertheless, the two populations differed in the mode that they use these resources, thus suggesting a possible phenotypic difference between them. Physiological responses of these isolates to nitrogen and phosphorus impoverishment seem to be more related to the type of limiting nutrient than to saprobity.
Similar content being viewed by others
Abbreviations
- α:
-
Photosynthetic efficiency
- Pmax :
-
Maximum photosynthetic rate
- Ik :
-
Saturation parameter
- β:
-
Photoinhibition parameter
- EQY:
-
Effective quantum yield
- ETR:
-
Electron transport rate
References
Andria JR, Vergara JJ, Llorens LP (1999) Biochemical responses and photosynthetic performance of Gracilaria sp. (Rhodophyta) from Cádis, Spain, cultured under different inorganic carbon and nitrogen levels. Eur J Phycol 34:497–504
Bautista AIN, Necchi Jr O (2007) Photoacclimation in three species of freshwater red algae. Braz J Plant Physiol 19:23–34
Beer S, Eshel A (1985) Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Aust J Mar Freshw Res 36:785–792
Biggs BJF, Stevenson RJ, Lowe RL (1998) A habitat matrix conceptual model for stream periphyton. Arch Hydrobiol 143:21–56
Bird KT, Habig C, DeBusk T (1982) Nitrogen allocation and storage patterns in Gracilaria tikvahiae (Rhodophyta). J Phycol 18:344–348
Brooks A (1986) Effects of phosphorus nutrition on ribulose-1,5-biphosphate carboxylase activation, photosynthetic quantum yield and amounts of Calvin-cycle metabolites in spinach leaves. Aust J Plant Physiol 13:221–237
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
Codispoti LA (1989) Phosphorus vs. nitrogen limitation of new and export production. In: Berger WH, Smetacek VS, Wefer G (eds) Productivity of the ocean: present and past. Wiley, New York, pp 377–394
Conde-Álvarez RM, Peres-Rodríguez E, Altamirano M, Nieto JM, Abdala R, Figueroa FL, Flores-Moya A (2002) Photosynthetic performance and pigment content in the aquatic liverwort Riella helicophylla under natural solar irradiance and solar irradiance without ultraviolet light. Aquat Bot 73:47–61
Dawes CJ, Kock EU (1990) Physiological responses of the red algae Gracilaria verrucosa and. G. tikvahiae before and after nutrient enrichment. Bull Mar Sci 46:335–344
Dring MJ (1990) Light haversting and pigment composition in marine phytoplankton and macroalgae. In: Herring PJ, Campbell AK, Whitfield M, Maddock L (eds) Light and life in the sea. Cambridge Univ Press, Cambridge, pp 89–103
Falkowski PG, LaRoche J (1991) Acclimation to spectral irradiance in algae. J Phycol 27:8–14
Falkwoski PG, Barber RT, Smetacek V (1998) Biogeochemical controls and feedbacks on ocean primary productivity. Science 281:200–206
Geider RJ, Macintyre HL, Graziano LM, McKay RML (1998) Responses of photosynthetic apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorus limitation. Eur J Phycol 33:315–332
Grime JP (1977) Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194
Grime JP (1979) Plant strategies and vegetation processes. Wiley, New York
Grossman AR, Schaffer MR, Chiang GG, Collier JL (1993) The phycobilisome, a light-harvesting complex response to environmental conditions. Microbiol Rev 57:725–749
Kaczmarczyk D, Sheath RG (1992) Pigment content and carbon to nitrogen ratios of freshwater red algae growing at different light levels. Jpn J Phycol 40:279–282
Kain JM (1987) Seasonal growth and photoinhibition in Plocamium cartilagineum (Rhodophyta) off the Isle of Man. Phycologia 32:401–409
Lapointe BE (1981) The effects of light and nitrogen on growth, pigment content, and biochemical composition of Gracilaria foliifera v. angustissima (Gigartinales, Rhodophyta). J Phycol 17:90–95
Lapointe BE, Duke CS (1984) Biochemical strategies of growth of Gracilaria tikvahiae (Rhodophyta) in relation to light intensity and nitrogen availability. J Phycol 20:488–495
Littler MM, Arnold KE (1985) Electrodes and chemicals. In: Littler MM, Littler DS (eds) Handbook of phycological methods; ecological field methods: macroalgae. Cambridge University Press, Cambridge, pp 349–375
MacArthur RH, Wilson EO (1967) Theory of island biogeography. Princeton University, Princeton
Mourthé CA Jr (2000) Modificações estruturais na comunidade de diatomáceas em um gradiente de poluição hídrica: trecho superior do Rio das Velhas (Região Metropolitana de Belo Horizonte, MG). Dissertação Univ Fed, Belo Horizonte
Necchi Jr O, Zucchi MR (2001) Photosynthetic performance of freshwater Rhodophyta in response to temperature, irradiance, pH and diurnal rhythm. Phycolog Res 49:305–318
Necchi Jr O, Branco LHZ, Dip MR (1994) Uso de macroalgas para avaliação da poluição orgânica no Rio Preto, noroeste do estado de São Paulo. Ann Acad Bras Cienc 66:359–371
Necchi Jr O, Branco CCZ, Gomes RRV (1999) Microhabitat and plant structure of compsopogon coeruleus (compsopogonaceae, rhodophyta) populations in streams from from São Paulo state, southeastern Brazil. Cryptogam Algol 20:75–87
Necchi Jr O, Fo GAS, Salomaki ED, West JA, Aboal M, Vis ML (2013) Global sampling reveals low genetic diversity within Compsopogon (Compsopogonales, Rhodophyta). Eur J Phycol 48:152–162
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Roberts J (1997) Control of supply line. Science 278:2073–2074
Sabater S, Aboal M, Cambra J (1989) Nuevas observaciones de rodofíceas en aguas epicontinentales del NE y SE de España. Limnética 5:93–100
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM (eds) Ecophysiology of photosynthesis, vol 100. Springer-Verlag, Berlim, pp 49–70
Sharkey TD (1990) Feedback limitation of photosynthesis and the physiological role of ribulose biphosphate carboxylase carbamylation. Bot Mag Tokyo Spec Tissue 2:87–105
Sladecek V (1974) System of quality from the biological point of view. Arch Hydrobiol Beih Ergevn Limnol 7:1–218
Thomas MLH (1988) Photosynthesis and respiration of aquatic macroflora using the light and dark bottle oxygen method and dissolved oxygen method and dissolved oxygen analyzer. In: Lobban CS, Chapman DJ, Kremer BP (eds) Experimental phycology. Cambridge University Press, Cambridge, pp 64–77
Tomas X, Lopez P, Margalef-Mir R, Comin FA (1980) Distribution and ecology of Compsopogon coeruleus (Balbis) Montagne in eastern Spain. Cryptog Algolog 3:179–186
Van Kooten O, Snel JJH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Vergara JJ, Niell X (1993) Effects of nitrate availability and irradiance on internal nitrogen constituents in Corallina elongata (Rhodophyta). J Phycol 29:285–293
Watanabe MM (2005) Freshwater culture media. In: Andersen RA (ed) Algal culture techniques. Elsevier Academic Press, Amsterdam, pp 13–20
Wetzel RG (1983) Limnology, 2nd edn. Saunders College, Philadelphia
Wetzel RG, Likens GE (2000) Limnlogical analyses. Springer-Verlag, New York
White AJ, Critchley C (1999) Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth Res 59:63–72
Zucchi MR, Necchi Jr O (2001) Effects of temperature, irradiance and photoperiod on growth and pigment content in some freshwater red algae in culture. Phycolog Res 49:103–114
Acknowledgments
This research was supported by a FAPESP (Fundação de Amparo à Pesquisa de São Paulo) master’s scholarship to AINB (05/03511-0); laboratory assistance by Maria Helena Carabolante is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bautista, A.I.N., Necchi-Júnior, O. Physiological performances of two populations of Compsopogon caeruleus (Rhodophyta) to inorganic nitrogen and phosphorus impoverishment. Braz. J. Bot 37, 391–398 (2014). https://doi.org/10.1007/s40415-014-0088-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-014-0088-8