Abstract
The National Institute for Health and Care Excellence (NICE) invited the manufacturer (Roche) of pralsetinib (Gavreto®), as part of the single technology appraisal (STA) process, to submit evidence for the clinical effectiveness and cost effectiveness of pralsetinib for the treatment of adult patients with rearranged during transfection (RET) fusion-positive advanced non-small-cell lung cancer (NSCLC) not previously treated with a RET inhibitor. Kleijnen Systematic Reviews Ltd, in collaboration with University Medical Center Groningen, was commissioned to act as the independent Evidence Review Group (ERG). This paper summarizes the company submission (CS), presents the ERG’s critical review of the clinical and cost-effectiveness evidence in the CS, highlights the key methodological considerations, and describes the development of the NICE guidance by the Appraisal Committee. The CS reported data from the ARROW trial. ARROW is a single-arm, multicenter, non-randomized, open-label, multi-cohort study in patients with RET fusion-positive NSCLC and other advanced solid tumors. The CS included both untreated and pre-treated RET fusion-positive NSCLC patients, among other disease types. The comparators in the untreated population were pembrolizumab + pemetrexed + chemotherapy and pembrolizumab monotherapy. The comparators for the pre-treated population were docetaxel monotherapy, docetaxel + nintedanib, and platinum-based chemotherapy ± pemetrexed. As no comparators were included in ARROW, an indirect treatment comparison was conducted to estimate relative effectiveness. The ERG’s concerns included the immaturity of data, small sample size, and lack of comparative safety evidence. The ERG considers the clinical evidence presented to be insufficiently robust to inform the economic model. Even when all the ERG preferred assumptions were implemented in the model, uncertainty remained on a number of issues, such as the appropriateness of the hazard ratios and the methods and data used to derive them, long-term efficacy of pralsetinib, and direct evidence for health-related quality of life (HRQoL). NICE did not recommend pralsetinib within its marketing authorization for treating RET fusion-positive advanced non-small-cell lung cancer (NSCLC) in adults who have not had a RET inhibitor before. The uncertainty of the clinical evidence and the estimates of cost effectiveness were too high to be considered a cost-effective use of NHS resources. Therefore, pralsetinib was not recommended for routine use.
Similar content being viewed by others
Although the clinical evidence suggested that pralsetinib could be clinically effective, National Institute for Health and Care Excellence (NICE) did not recommend pralsetinib within its marketing authorization for treating rearranged during transfection (RET) fusion-positive advanced non-small-cell lung cancer (NSCLC) in adults who have not had a RET inhibitor before. This decision was made because of uncertainties in various elements as listed below. |
There was a mismatch between the NICE final scope and the evidence provided in the company submission where it concerned population and comparators. The appraisal population was restricted to non-squamous NSCLC, whereas the population defined in the final NICE scope includes all patients with NSCLC. In addition, the comparators were not in line with the final NICE scope and the company relied on clinical expert opinion instead of objective evidence as to actual clinical practice. |
The absence of comparative safety data for pralsetinib versus comparators listed in the NICE final scope made it impossible to draw firm conclusions regarding the relative safety and tolerability of pralsetinib. |
Treatment benefit over time was modeled using constant hazard ratios, derived from indirect treatment comparisons and immature trial data. The cumulative uncertainty caused by these factors is difficult to quantify and causes problems for decision making in technology appraisals. |
1 Introduction
Pralsetinib (trade name Gavreto®) was appraised within the National Institute for Health and Care Excellence (NICE) single technology appraisal (STA) process. Health technologies must be shown to be clinically effective and to represent a cost-effective use of National Health Service (NHS) resources in order to be recommended by NICE. Within the STA process, the company (Roche) provided NICE with a written submission and a mathematical health economic model, summarizing the company’s estimates of the clinical and cost effectiveness of pralsetinib as monotherapy for the treatment of adult patients with rearranged during transfection (RET) fusion-positive advanced non-small-cell lung cancer (NSCLC) not previously treated with a RET inhibitor. This company submission (CS) was reviewed by an Evidence Review Group (ERG) independent of NICE [1]. The ERG, Kleijnen Systematic Reviews in collaboration with Groningen University Medical Center, produced an ERG report [1]. After consideration of the evidence submitted by the company and the ERG report, the NICE Appraisal Consultation Document (ACD) issued guidance whether or not to recommend the technology by means of the Final Appraisal Document (FAD), which was open for appeal. This paper presents a summary of the ERG report and the development of the NICE guidance. Furthermore, it highlights important methodological issues which may help in future decision making.
Full details of all relevant appraisal documents (including the appraisal scope, CS, ERG report, consultee submissions, ACD, FAD, and comments from consultees) can be found on the NICE website [1].
2 The Decision Problem
The CS defined the population as adult patients with RET fusion-positive advanced NSCLC not previously treated with a RET inhibitor, further categorized into two subgroups: untreated and previously treated (having had systemic treatment before) [2, 3]. The comparators included in the CS for the untreated population were pembrolizumab + pemetrexed + chemotherapy and pembrolizumab monotherapy. The comparators for the pre-treated population were docetaxel monotherapy, docetaxel + nintedanib, and platinum-based chemotherapy ± pemetrexed.
3 Independent Evidence Review Group (ERG) Review
The ERG reviewed the clinical-effectiveness and cost-effectiveness evidence of pralsetinib for this indication. As part of the STA process, the ERG and NICE had the opportunity to ask for clarification on specific issues in the CS, in response to which the company provided additional information [4]. Based on this information, the ERG produced an ERG base case by modifying the health economic model submitted by the company, and assessed the impact of alternative assumptions and parameter values on the model results. Sections 3.1–3.7 summarize the evidence presented in the CS, as well as the review of the ERG.
3.1 Critique of the Decision Problem as Defined By the Company Submission (CS)
The ERG identified two main issues with the decision problem: the appraisal evidence used to inform the model inputs was restricted to non-squamous NSCLC, whereas the population defined in the decision problem by the company included all patients with RET fusion-positive advanced NSCLC not previously treated with a RET inhibitor; the comparators were not in line with the final NICE scope, leaving the relative benefits of pralsetinib unclear. Numerous comparators listed in the NICE final scope were omitted from the CS, including
-
for untreated patients: pembrolizumab with carboplatin and paclitaxel, atezolizumab monotherapy, nivolumab plus ipilimumab, chemotherapy (gemcitabine or vinorelbine) in combination with a platinum drug (carboplatin or cisplatin), and pembrolizumab with carboplatin and paclitaxel;
-
for treated patients: atezolizumab monotherapy, pembrolizumab monotherapy, and best supportive care.
The company justified the restriction to non-squamous carcinoma according to the low proportion of patients with squamous cell histology in their trial: “Due to the low incidence of RET fusion-positive squamous patients and the small number of squamous patients in ARROW, it was not deemed suitable or feasible to include this population; therefore this appraisal is concentrated solely on non-squamous NSCLC patients,…” (p. 14, CS) [2]. Despite this limitation in the evidence, the company argued that the population in the decision problem should include squamous histology, largely on the basis of precedent, that is, the previous NICE appraisal of selpercatinib, TA760: “…Roche believes the appraisal population should be all encompassing including squamous patients (in line with the expected license) rather than restricted to non-squamous, as per the selpercatinib appraisal” [5, 6]. The ERG concluded that precedent was insufficient justification: given the lack of evidence, it was possible that pralsetinib might not be clinically effective let alone cost effective in patients with squamous histology.
The NICE clinical expert did not agree with the omission of atezolizumab monotherapy first line, and the ERG noted that a complete justification for omission of best supportive care was missing [7].
The decision problem also differed from the final scope with the addition of precluding prior RET inhibitor in line with the marketing authorization. However, this was not identified as an issue by the ERG given that at the time of the appraisal no RET inhibitor was used in the NHS. Currently, selpercatinib is not recommended for routine commissioning but only for use in the Cancer Drugs Fund (CDF) as part of NICE TA760 in January 2022 [6].
3.2 Clinical Effectiveness Evidence Submitted by the Company
The CS reported data from the ARROW trial. The ARROW trial is a phase I/II, single-arm, multicenter, non-randomized, open-label, multi-cohort study in patients with RET fusion-positive NSCLC and other advanced solid tumors [8]. The study included a phase I dose escalation part to determine the maximum tolerated dose (MTD) and recommended phase II dose (RP2D) of pralsetinib, followed by a phase II expansion part to assess the clinical efficacy of pralsetinib in specific tumor types and treatment settings. Phase I was completed with 62 patients (58 from the US, 4 from Europe). Phase II dose expansion is ongoing in 79 centers and 13 countries: Belgium, China, France, Germany, Hong Kong, Italy, South Korea, the Netherlands, Singapore, Spain, Taiwan, the UK, and the US. Only 13 UK patients were included in this trial, which may limit generalizability to the UK clinical setting.
The primary efficacy endpoint of the ARROW phase II trial was objective response rate (ORR). ORR was defined as the proportion of patients with a confirmed response—complete response (CR) or partial response (PR)—for at least two assessments at least 28 days apart and no progressed disease (PD) in between. The secondary efficacy endpoints of the ARROW phase II trial included duration of response (DOR), clinical benefit rate (CBR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS) outcomes.
ORR in patients with RET fusion-positive NSCLC treated with 400 mg QD (quaque die, i.e., every day) (n = 216) was 68.5% (95% confidence intervals [CI]: 61.9–74.7). ORR results were similar among patients in this population irrespective of prior treatment (treatment-naïve subgroup [n = 68]: ORR 79.4% [95% CI 67.9–88.3], prior systemic treatment subgroup [n = 148]: ORR 63.5% [95% CI 55.2–71.3]) [9]. Median PFS was 16.4 months (n = 233) (95% CI 11.0–24.1) after a median follow-up of 18.4 months. PFS for the treatment-naïve subgroup and the prior systemic treatment subgroup was 13.0 (95% CI 9.1–NR) and 16.5 (95% CI 10.5–24.1) months, respectively. Among all 281 patients in the unrestricted efficacy population, median OS was not reached (95% CI NR–NR) after median follow-up of 17.1 months. OS for the treatment-naïve subgroup and the prior systemic treatment subgroup was not reached after median follow-up of 12.8 months (95% CI 11.1–15.0) and 20.1 months (95% CI 19.4–21.5), respectively [10].
For RET fusion-positive NSCLC, (n = 281), 94% of patients (n = 264) had treatment-related adverse events (TRAEs). Specific TRAEs included increased aspartate aminotransferase (AST) (40.6%), anemia (35.9%), increased alanine aminotransferase (ALT) (29.6%), neutrophil count decreased (28.1%), constipation (26%), hypertension and white blood cell (WBC) count decreased (24.9% each). There was no comparison between pralsetinib and comparators for safety outcomes; nevertheless, the company concluded that: “…pralsetinib was well tolerated at a dose of 400 mg once daily in patients with RET fusion-positive NSCLC.” [2].
3.3 Critique of Clinical Effectiveness Evidence and Interpretation
The ERG identified three major concerns with the clinical effectiveness evidence. Firstly, there were only 13 UK patients included in the ARROW trial, which might limit generalizability to the UK population [8]. Secondly, the systematic literature reviews (SLRs) upon which the estimations of a treatment effect between pralsetinib and each comparator of interest were based suffered from methodological problems including inconsistency of response rate definitions, lack of dual independent data extraction, unclear eligibility criteria, exclusion of non-randomized studies, and lack of comprehensive quality assessment of included studies. This hindered the ERG’s ability to draw robust conclusions about the safety and effectiveness of pralsetinib. Thirdly, there are no safety data available for pralsetinib versus the comparators listed in the NICE Final Scope for the treatment of patients with advanced, unresectable, RET-altered NSCLC, due to evidence from a single-arm study and no attempt at indirect comparison.
3.4 Cost-Effectiveness Evidence Submitted by the Company
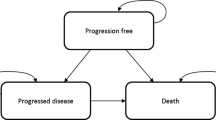
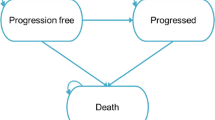
The company conducted SLRs to identify relevant economic, cost and resource use, and HRQoL evidence. The company built a de novo economic model using a partitioned survival model (PSM). The model comprised three health states, i.e. PFS, PD, and death.
The model adopted the perspective of the NHS and Personal Social Services. The model time horizon was 25 years, with a cycle length of 1 month. A half-cycle correction was applied. All costs and quality-adjusted life-years (QALYs) were discounted at a rate of 3.5% per year.
The patient population included in the economic evaluation consisted of adult patients with RET fusion-positive advanced NSCLC not previously treated with a RET inhibitor. This is in line with the marketing authorization. The marketing authorization is line-agnostic, meaning patients are eligible to be treated with pralsetinib in all lines of treatment. Pralsetinib was included in the model as per the licensed dosing regimen (administered 400 mg orally QD until disease progression or unacceptable toxicity). The primary comparator in the untreated analysis was pembrolizumab + pemetrexed + chemotherapy with a secondary analysis against pembrolizumab monotherapy. The primary comparator for the pre-treated economic evaluation was docetaxel monotherapy with secondary analyses against docetaxel + nintedanib and an additional analysis provided against platinum-based chemotherapy ± pemetrexed.
The primary source for clinical data for pralsetinib in the economic model was the ARROW study [8]. Given that the ARROW trial is a single-arm study, the company did an indirect treatment comparison to estimate the relative effectiveness of pralsetinib compared with other treatments. Results for OS, PFS, and time to treatment discontinuation (TTD) results from ARROW were extrapolated to the time-horizon of the model. The company used parametric models to extrapolate survival for both the untreated and previously treated subgroups. The extrapolations were uncertain because of the immaturity of the data and the small sample size. The overall median follow-up in ARROW was just over 21.5 months for untreated patients. For the untreated sub-group, hazard ratios were estimated from a comparison of untreated pralsetinib patients in ARROW to untreated advanced NSCLC patients receiving the comparator treatment in the US Flatiron Health dataset [11, 12]. Patients in comparator arms were adjusted using propensity score matching based on baseline characteristics to adjust for differing characteristics of RET fusion-positive patients. However, the comparison with platinum-based chemo ± pemetrexed in the pre-treated sub-group was not propensity score matched. For the pre-treated sub-group, hazard ratios were estimated from comparing pre-treated pralsetinib patients in ARROW to available published studies of advanced NSCLC patients. Patients in the comparator arms were adjusted based on baseline characteristics to adjust for differing characteristics of RET fusion-positive patients where possible. Survival for the comparators in both treated and untreated sub-groups was modeled by applying a hazard ratio from the indirect treatment comparison to the modeled pralsetinib OS, PFS, and TTD.
EuroQol 5 Dimensions (EQ-5D) data was not collected in ARROW. Rather, the European Organization for Research and Treatment of Cancer quality of life questionnaire (EORTC QLQ-C30) was used to obtain HRQoL data, collected directly from RET fusion-positive NSCLC patients. The company explored the feasibility of mapping from EORTC QLQ-C30 to EQ-5D-3L in order to inform utilities for the economic model that were informed by ARROW clinical trial data. However, given the large volume of missing data, utilities derived in this way were not considered robust enough to inform decision making. Therefore, health state utility values from the literature were preferred. Due to the paucity of health state utility value data in the population of interest, previous NICE appraisals were hand searched in order to identify the most relevant health state utility values to inform the current economic model. Given the similarities between the current appraisal and ID3743 on selpercatinib for previously treated RET fusion-positive advanced NSCLC, the company chose to include health state utility values proposed in TA760 in this appraisal [6]. In the absence of RET fusion-positive health state utility data, it was assumed that RET fusion-positive patients do not demonstrate different HRQoL from advanced NSCLC patients and therefore advanced NSCLC health state utility values can be used.
For medicines available to the NHS as generic medicines, prices were taken from the electronic market information tool (eMIT), which reports the average price paid by the NHS for a generic medicine for the last period [13]. For medicines only available to the NHS as proprietary medicines, prices were taken as the list price stated in the British National Formulary (BNF) [14]. All other treatments were assumed to be at list price. For regimens including either cisplatin or carboplatin, a 50:50 split of cisplatin and carboplatin was assumed. For pre-treated treatment with platinum-based chemotherapy ± pemetrexed, no other platinum-based chemotherapies were included in the costings given the minimal impact of differences in acquisition costs of platinum-based chemotherapies on model results and that cisplatin/carboplatin are the most commonly used. Further, for platinum-based chemotherapy ± pemetrexed, it was assumed that 63% of patients received pemetrexed. Drug administration costs were extracted from NHS reference costs [15]. Supportive care costs were applied for both PF and PD health states. All unit costs were derived from NHS reference costs and the Personal Social Services Research Unit (PSSRU) [14, 16]. The cost of adverse events for each treatment arm was calculated by multiplying the incidence of each adverse event and its unit cost. Adverse event costs were applied as a one-off cost during the first cycle of treatment only, assuming that the adverse event occurs at treatment initiation, only once across the time horizon of the model. The economic model includes costs and resource use of subsequent treatment for patients who have progressed after first-line treatment with pralsetinib, or the relevant comparators. Subsequent treatment costs were applied as a one-off cost in the economic model when patients enter the PD health state.
As the incremental cost-effectiveness ratios (ICERs) resulting from the model are commercial-in-confidence because of confidential discounts for pralsetinib and its comparators, the ICERs cannot be reported here. The FAD states: “NICE's guide to the methods of technology appraisal notes that above a most plausible ICER of £20,000 per QALY gained, judgements about the acceptability of a technology as an effective use of NHS resources will take into account the degree of certainty around the ICER and whether the technology meets the criteria for consideration as a ‘life-extending treatment at the end of life’. The committee noted the uncertainties informing the cost-effectiveness estimates, including the primary clinical evidence being from a single-arm trial, limitations with the indirect treatment comparisons and a constant treatment benefit for pralsetinib applied throughout the modeled time horizon. Because of these uncertainties, the committee considered the maximum acceptable ICER would be at the lower end of the range normally considered a cost-effective use of NHS resources.” Therefore, although the ICER is confidential, it must be above £20,000. Overall, the technology was modeled to affect QALYs by increasing PFS and OS in the pralsetinib arm relative to its comparators. In addition, the technology was modeled to affect costs by higher monthly cost of treatment for pralsetinib compared with the majority of comparator treatments. Moreover, the cost was affected by the oral administration of pralsetinib, instead of IV administration for comparator treatments and a higher proportion of patients receiving subsequent treatment after first-line treatment of pralsetinib, relative to the comparator treatments. The company performed and presented the results of probabilistic sensitivity analyses (PSA), deterministic sensitivity analyses (DSA), as well as scenario analyses. Notably, there was a substantial difference between deterministic and probabilistic ICERs, mainly for the untreated population. The company was not able to explain this difference.
3.5 Critique of Cost-Effectiveness Evidence and Interpretation
The ERG had concerns about the appropriateness of the PSM. Ideally, the results would be verified by a different type of model, such as a state transition model. The potential issue with PSMs is that PFS and TTD can potentially exceed OS when independently sampled. Moreover, the ERG was concerned about the exclusion of atezolizumab in the pre-treated population. The NHS CDF clinical lead advised that atezolizumab is a relevant comparator for first-line pralsetinib [7]. Besides the choices for the model and comparators, the ERG was concerned about the extrapolation of the data.
Due to the immaturity of the data and small differences in fit-related statistics, base-case and scenario curves for extrapolation were not chosen based on best statistical fit. Instead, curve choices were made using available landmark survival point predictions provided by clinical experts. Although some of the curve choices were on the conservative side for pralsetinib, the underprediction of survival for comparator curves was often even larger, both in absolute and relative terms. Similar trends were observed with some of the other curve selections. However, it was difficult to identify curves that were optimal for both pralsetinib and comparators, in particular for the untreated population. Moreover, the company did not include a treatment waning effect but assumed that the pralsetinib and comparator curves for OS and PFS would remain separated for the entire time horizon, until mortality of the general population would take over. Since the hazard ratios applied by the company were based on small sample size and immature data, in particular for the untreated population which was a smaller group in ARROW and had a median follow-up of 9.5 months, a constant and unending treatment effect seems unrealistic. After the consultation stage, the company updated their model by removing the proportional hazards assumption for a number of comparators and fitting the survival curves independently. The ERG considered this an improvement to the model but noted that a constant treatment effect was still seen over the full time horizon of the model, while a solid basis for this was still lacking.
Although EORTC-QLQ-C30 data were available from the ARROW trial, the company considered these unfit to inform the economic model. The company therefore chose to use health state utilities from previous STAs. In particular for the untreated population, the ERG was not convinced that the STA chosen to inform the base-case (from an epidermal growth factor receptor [EGFR]-positive population) was indeed the most suitable proxy, as the two STAs in the scenarios (anaplastic lymphoma kinase [ALK] and ROS proto-oncogene 1, receptor tyrosine kinase [ROS1] positive populations) were also said to be suitable proxies [2, 17, 18]. Moreover, there was a lack of justification for the difference in health state utilities between the untreated and pre-treated populations. The base-case utilities were substantially higher for the untreated compared with the pre-treated population. In the clarification phase, the ERG asked the company to provide a justification for this difference, and also requested the mapped EORTC QLQ-C30 data from ARROW, stratified for population. In their response, the company stated that those who were progression-free in the pre-treated population would, in terms of HRQoL, be comparable to those after progression in the untreated population. However, the mapped EORTC QLQ-C30 data revealed that there was hardly any difference between the two populations, and the pre-treated population had a higher mapped utility value even than the untreated population. Since the ARROW data are the only source of evidence that includes both untreated and pre-treated populations in one dataset, and are RET-positive specific, the ERG is concerned about the validity of the utility scores used in the company base-case, not informed by the ARROW trial and not coming from the same source.
The ERG was concerned about the assumption of health state costs being equal between untreated and pre-treated populations. The company confirmed their view of the pre-treated population being less healthy and therefore it would be expected that resource use would also be higher compared with the untreated population. The resource use data was sourced from previous line-agnostic appraisals and so probably the resource use for the untreated population would in reality be slightly lower, and for the pre-treated it may be slightly higher. Given that health state costs can account for at least 20% of total costs (depending on line and comparator), changing resource use could have an impact on the ICER. Moreover, the ERG was concerned about the assumption of 100% relative dose intensity (RDI) for all treatments. In a previous STA, RDI was around 90% for all included treatments as proposed by the company in their submission [19].
3.6 Additional Work Undertaken by the ERG
Based on all considerations highlighted in the ERG critique, the ERG defined a new base case, in which various adjustments were made to the company’s base case. This included correction of the model to ensure that OS could not fall below PFS or TTD in the PSA and the correction of cisplatin doses in the second line to 75 mg/m2 instead of 20 mg Q3W (once every 3 weeks). Moreover, the ERG added treatment waning of OS, assuming treatment waning starting at 2 years, decreasing to an HR of 1 over a period of 3 years.
Furthermore, the ERG explored a more extreme version of the ERG preferred assumption of treatment waning starting at 1 year, decreasing over a period of 2 years. Additionally, the ERG explored the effect of adjusted hazard ratios, calibrated to expert opinion estimates at the 3-year mark for OS and PFS. The ERG explored the scenarios of assuming treatment duration equal to PFS and assuming relative dose intensity of 90% for all treatments to test robustness of model results to potential dose reductions in clinical practice.
3.7 Conclusions of the ERG Report and Technical Engagement
The cost-effectiveness estimates of pralsetinib in the untreated sub-group are subject to considerable uncertainty, mainly because of immaturity of data, small sample size, and lack of comparative evidence in various areas. The ERG considers the clinical evidence presented to be not sufficiently robust to inform the economic model. Even when all the ERG preferred assumptions were implemented in the model, uncertainty remained on a number of issues, such as the appropriateness of the hazard ratios and the methods and data used to derive them, long-term efficacy of pralsetinib, and comparative HRQoL values. In the pre-treated sub-group, these uncertainties are present as well, but the ICERs for the pre-treated sub-group comparisons are well outside the cost-effective range, and therefore the uncertainty has less of an impact on decision making.
4 Key Methodological Issues
The population in the company submission evidence was limited to patients with non-squamous cell NSCLC, while the population defined in the decision problem and the NICE scope included squamous histology. Studies in this narrower population may not apply to the whole population in this setting. The ERG recommended that the decision problem should have been modified to reflect the narrower population.
Numerous comparators listed in the NICE final scope were omitted from the company submission, and the NICE clinical expert did not agree with some of these omissions. The ERG also noted that a complete justification for omission of best supportive care was missing. Moreover, the ERG noted that choice of comparator should not be determined by clinical expert opinion given that this might vary, and instead, objective treatment pattern evidence should be employed.
There was no comparison of safety data for pralsetinib versus the comparators listed in the NICE Final Scope for the treatment of patients with advanced, unresectable, RET-altered NSCLC. Adverse event rates were incorporated in the economic model from various literature sources, but the ERG argued that a formal comparison should be made. Without this, conclusions about the safety and effectiveness of pralsetinib are severely hindered.
The company assumed that the benefit of pralsetinib was constant over time, even though the evidence from ARROW was insufficient to justify this and the company did not justify excluding treatment waning. The small sample size and immaturity of data from the ARROW trial, in particular in the untreated population, resulted in substantial uncertainty in the estimated hazard ratios and the survival curve extrapolations. The ERG preferred to calibrate the hazard ratios in a scenario so that both pralsetinib and comparator curves are best aligned with the expert estimates.
Mapped utilities from the ARROW study were disqualified by the company and instead, utility values from previous appraisals were used to inform the economic model. These were, however, not specific to the RET-fusion positive population and the difference in utility scores between the untreated and pre-treated population was not reflected in the mapped EORTC QLQ-C30 data from the ARROW study.
The key differences between the company’s preferred assumptions and the ERG’s preferred assumptions were the correction to prevent OS, PFS, and TTD curves from crossing in the PSA and the implementation of a treatment waning effect. In a scenario, the ERG explored alternative hazard ratios to account for substantial uncertainty surrounding these. In general, changing the company assumptions increased the ICER of pralsetinib relative to the comparator treatments.
5 National Institute for Health and Care Excellence Guidance
On 3 August 2022, NICE did not recommend pralsetinib within its marketing authorization for treating RET fusion-positive advanced non-small-cell lung cancer (NSCLC) in adults who have not had a RET inhibitor before.
5.1 Consideration of Clinical Effectiveness
The clinical evidence for pralsetinib suggests it could be clinically effective. However, the benefit is uncertain because pralsetinib was not compared directly with any usual NHS treatments. The results from indirectly comparing pralsetinib with some usual treatments were uncertain.
5.2 Consideration of Cost Effectiveness
Pralsetinib meets NICE's criteria to be a life-extending treatment at the end of life for people with previously treated NSCLC, but not for untreated NSCLC. The committee concluded that the assumption of pralsetinib's constant benefit over time may be implausible, particularly because there is no trial evidence beyond 18 months. The committee noted the uncertainties informing the cost-effectiveness estimates, including the primary clinical evidence being from a single-arm trial, limitations with the indirect treatment comparisons and a constant treatment benefit for pralsetinib applied throughout the modeled time horizon. Because of these uncertainties, the committee’s preferred cost-effectiveness estimates were above the maximum ICERs considered a cost-effective use of NHS resources for the untreated and treated groups. Therefore, pralsetinib was not recommended for routine use.
6 Conclusions
This article describes the STA considering pralsetinib for adult patients with RET fusion-positive advanced NSCLC not previously treated with a RET inhibitor.
This STA illustrates the difficulty with the NICE Technical Support Document recommendation that ideally, mature data should be provided to verify the plausibility of extrapolations of the OS, PFS, and TTD [20]. This recommendation is very rarely put into practice. In this appraisal, the immature data, the indirect comparison of pralsetinib with comparators and the absence of some relevant comparators have played a major role in the recommendation of the committee. Because of the uncertainty in the clinical evidence, the estimates of cost effectiveness are uncertain and too high to be considered a cost-effective use of NHS resources. Therefore, pralsetinib was not recommended for routine use.
References
National Institute for Health and Care Excellence. Pralsetinib for treating RET fusion-positive advanced non-small-cell lung cancer: NICE Technology appraisal guidance 812. Evidence [Internet]. London: NICE; 2022. https://www.nice.org.uk/guidance/ta812/evidence. Accessed 8 Dec 2022.
Roche Products Ltd. Pralsetinib for RET fusion-positive advanced non-small-cell lung cancer [ID3875]. Document B. Company evidence submission. National Institute of Health and Care Excellence. Single technology appraisal (STA). Roche, 2021.
National Institute for Health and Care Excellence. Pralsetinib for RET fusion-positive advanced non-small-cell lung cancer [ID3875]. Health Technology Appraisal. Final scope [Internet]. London: National Institute for Health and Care Excellence; 2021. https://www.nice.org.uk/guidance/gid-ta10770/documents/final-scope. Accessed 16 Aug 2021.
National Institute for Health and Care Excellence. Pralsetinib for RET fusion-positive advanced non-small-cell lung cancer [ID3875]: committee papers [Internet]. London: National Institute for Health and Care Excellence; 2022. https://www.nice.org.uk/guidance/ta812/documents/committee-papers. Accessed 8 Dec 2022.
Roche. Pralsetinib for RET fusion-positive advanced non-small-cell lung cancer [ID3875]. Clarification questions response. Roche, 2021.
National Institute for Health and Care Excellence. Selpercatinib for RET fusion-positive advanced non-small-cell lung cancer. Appraisal consultation document [Internet]. London: National Institute for Health and Care Excellence; 2021. https://www.nice.org.uk/guidance/GID-TA10618/documents/129. Accessed 21 Sept 2021.
NHS CDF clinical lead. ID3875 pralsetinib for people with advanced RET fusion-positive non-small cell lung cancer—early NHS clinical insights. Clinical expert opinion [Personal communication]. [Personal].
Gainor JF, Curigliano G, Kim DW, Lee DH, Besse B, Baik CS, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22(7):959–69.
Curigliano G, Gainor JF, Griesinger F, Thomas M, Subbiah V, Baik CS, et al. Safety and efficacy of pralsetinib in patients with advanced RET fusion–positive non-small cell lung cancer: update from the ARROW trial. American Society of Clinical Oncology (ASCO) annual meeting; 4–8 Jun 2021. Online, 2021.
Griesinger F, Curigliano G, Thomas M, Subbiah V, Baik CS, Tan DSW, et al. Safety and efficacy of pralsetinib in RET fusion-positive non-small-cell lung cancer including as first-line therapy: update from the ARROW trial. Ann Oncol. 2022;33(11):1168–78.
Khozin S, Miksad RA, Adami J, Boyd M, Brown NR, Gossai A, et al. Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non-small cell lung cancer. Cancer. 2019;125(22):4019–32.
Cytel Inc. Effectiveness comparison between Flatiron EDM patients on three different regimes and ARROW RET-fusion positive patients given pralsetinib. Technical Report. Version 4.0. Prepared by Cytel Inc. for Sreeram Ramagopalan, F. Hoffmann-La Roche. Cytel Inc, 2021.
Department of Health and Social Care. Drugs and pharmaceutical electronic market information tool (eMIT) [Internet]. London: Department of Health and Social Care; 2021. https://www.gov.uk/government/publications/drugs-and-pharmaceutical-electronic-market-information-emit. Accessed 16 Aug 2021.
Joint Formulary Committee. British National Formulary [Internet]. London: BMJ Group and Pharmaceutical Press; 2021. https://bnf.nice.org.uk/. Accessed 16 Aug 2021.
NHS. National cost collection 2018/19 [Internet]. London: NHS; 2020. https://www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/. Accessed 16 Aug 2021.
Curtis LA, Burns A. Unit costs of health and social care 2020 [Internet]. Canterbury: Personal Social Services Research Unit (PSSRU), University of Kent; 2020. https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2020/. Accessed 16 Aug 2021.
National Institute for Health and Care Excellence. Afatinib for treating epidermal growth factor receptor mutation-positive locally advanced or metastatic non-small-cell lung cancer. NICE technology appraisal guidance 310 [Internet]. London: National Institute for Health and Care Excellence; 2014. https://www.nice.org.uk/guidance/ta310. Accessed 16 Aug 2021.
National Institute for Health and Care Excellence. Entrectinib for treating ROS1-positive advanced non-small-cell lung cancer. NICE technology appraisal guidance 643 [Internet]. London: National Institute for Health and Care Excellence; 2020. https://www.nice.org.uk/guidance/ta643. Accessed 16 Aug 2021.
National Institute for Health and Care Excellence. Sotorasib for previously treated KRAS G12C mutated, locally advanced or metastatic non-small-cell lung cancer ID3780. NICE technology appraisal guidance. In development [GID-TA10639] [Internet]. London: National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/indevelopment/gid-ta10639. Accessed 8 Oct 2021.
Latimer N. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. Last updated March 2013 [Internet]. Sheffield: NICE Decision Support Unit; 2011. http://www.nicedsu.org.uk. Accessed 10 Aug 2021.
Acknowledgements
This summary of the ERG report was compiled after NICE issued the FAD. All authors have commented on the submitted manuscript and have given their approval for the final version to be published. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of NICE or the Department of Health. Any errors are the responsibility of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (award ID NIHR135136). Please visit the HTA programme website for further project information (https://www.nihr.ac.uk/explore-nihr/funding-programmes/health-technology-assessment.htm).
Conflict of interest
MA, NA, JH, SO, PP, SR, CA, SK, MP, SD, RW, AvA have no conflicts of interest to declare.
Author contributions
All authors have commented on the submitted manuscript and have given their approval for the final version to be published. JH, PP, NA, and SO critiqued the clinical effectiveness data reported by the company. SD critiqued the literature searches undertaken by the company. MA, CA, SK, MP, NA, and AvA critiqued the mathematical model provided and the cost-effectiveness analyses submitted by the company. RW critiqued the company’s definition of the decision problem and their description of the underlying health problem and current service provision. AvA acts as overall guarantor for this article. This article has not been externally peer reviewed by PharmacoEconomics.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Al Khayat, M.N.M.T., Armstrong, N., Howick, J. et al. Pralsetinib for RET Fusion-Positive Advanced Non-small-Cell Lung Cancer: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. PharmacoEconomics 41, 353–361 (2023). https://doi.org/10.1007/s40273-023-01247-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-023-01247-w