Abstract
Objective
We estimated the cost consequence of Italian National Health System (NHS) investment in direct-acting antiviral (DAA) therapy according to hepatitis C virus (HCV) treatment access policies in Italy.
Methods
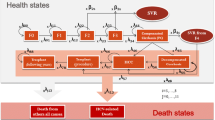
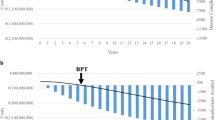
A multistate, 20-year time horizon Markov model of HCV liver disease progression was developed. Fibrosis stage, age and genotype distributions were derived from the Italian Platform for the Study of Viral Hepatitis Therapies (PITER) cohort. The treatment efficacy, disease progression probabilities and direct costs in each health state were obtained from the literature. The break-even point in time (BPT) was defined as the period of time required for the cumulative costs saved to recover the Italian NHS investment in DAA treatment. Three different PITER enrolment periods, which covered the full DAA access evolution in Italy, were considered.
Results
The disease stages of 2657 patients who consecutively underwent DAA therapy from January 2015 to December 2017 at 30 PITER clinical centres were standardized for 1000 patients. The investment in DAAs was considered to equal €25 million, €15 million, and €9 million in 2015, 2016, and 2017, respectively. For patients treated in 2015, the BPT was not achieved, because of the disease severity of the treated patients and high DAA prices. For 2016 and 2017, the estimated BPTs were 6.6 and 6.2 years, respectively. The total cost savings after 20 years were €50.13 and €55.50 million for 1000 patients treated in 2016 and 2017, respectively.
Conclusions
This study may be a useful tool for public decision makers to understand how HCV clinical and epidemiological profiles influence the economic burden of HCV.



Similar content being viewed by others
References
Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17(2):107–15.
World Health Organization. Global Hepatitis Report 2017. http://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf;jsessionid=B5D28826A9E2D7DEFC5A15956902F383?sequence=1. Accessed 10 Nov 2017.
World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. 2016. http://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf?sequence=1. Accessed 10 Nov 2017.
Razavi H, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57(6):2164–70.
Deuffic-Burban S, et al. Predicted effects of treatment for HCV infection vary among European countries. Gastroenterology. 2012;143(4):974–85.
Andriulli A, et al. Declining prevalence and increasing awareness of HCV infection in Italy: a population-based survey in five metropolitan areas. Eur J Intern Med. 2018;53:79–84.
European Centre for Disease Prevention and Control. Systematic review on hepatitis B and C prevalence in the EU/EEA. Stockholm: ECDC; 2016.
ISTAT. Le principali cause di morte in Italia. Anno 2012. https://www.istat.it/it/files/2014/12/Principali_cause_morte_2012.pdf. Accessed 15 Apr 2018.
Guadagnino V, et al. Prevalence, risk factors, and genotype distribution of hepatitis C virus infection in the general population: a community-based survey in southern Italy. Hepatology. 1997;26(4):1006–11.
Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–76.
Agenzia Italiana del Farmaco. 2018. www.aifa.gov.it. Accessed 15 May 2018.
Marcellusi A, et al. Early treatment in HCV: is it a cost-utility option from the Italian perspective? Clin Drug Investig. 2016;36(8):661–72.
Craxi L, et al. Prioritization of high-cost new drugs for HCV: making sustainability ethical. Eur Rev Med Pharmacol Sci. 2016;20(6):1044–51.
Paolini D, et al. Cost analysis of residual viremia detected by two real-time PCR assays for response-guided (dual or triple) therapy of HCV genotype 1 infection. Value Health. 2015;18(7):A587.
Ruggeri M, et al. Cost-effectiveness analysis of early treatment of chronic HCV with sofosbuvir/velpatasvir in Italy. Appl Health Econ Health Policy. 2018;16:711–22.
Mennini FS, et al. Health policy model: long-term predictive results associated with the management of hepatitis C virus-induced diseases in Italy. Clinicoecon Outcomes Res. 2014;6:303–10.
Kondili La, Vella S, PC Group. PITER: an ongoing nationwide study on the real-life impact of direct acting antiviral based treatment for chronic hepatitis C in Italy. Dig Liver Dis. 2015;47(9):741–3.
ISTAT. Life tables. 2018. http://dati.istat.it/Index.aspx?DataSetCode=DCIS_MORTALITA1. Accessed 1 Dec 2017.
Gardini I, et al. HCV: estimation of the number of diagnosed patients eligible to the new anti-HCV therapies in Italy. Eur Rev Med Pharmacol Sci. 2016;20(1 Suppl):7–10.
Fattore G, et al. Associazione Italiana di Economia Sanitaria. Proposta di linee guida per la valutazione economica degli interventi sanitari. Politiche sanitarie. 2009;10(2):91–9.
Rappaport A. The discounted payback period. Manag Serv. 1965;15:30–6.
Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford handbooks in health economic evaluation. Oxford: Oxford University Press; 2006. p. 237.
Cortesi PA, et al. Management of treatment-naive chronic hepatitis C genotype 1 patients: a cost-effectiveness analysis of treatment options. J Viral Hepat. 2015;22(2):175–83.
Linthicum MT, et al. Value of expanding HCV screening and treatment policies in the United States. Am J Manag Care. 2016;22(6 Spec No.):SP227–35.
Marcellusi A, et al. The economic burden of HCV-induced diseases in Italy. A probabilistic cost of illness model. Eur Rev Med Pharmacol Sci. 2015;19(9):1610–20.
Thomas DL, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284(4):450–6.
Benhamou Y, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–8.
Missiha SB, Ostrowski M, Heathcote EJ. Disease progression in chronic hepatitis C: modifiable and nonmodifiable factors. Gastroenterology. 2008;134(6):1699–714.
Tong MJ, et al. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463–6.
Wiese M, et al. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single-source outbreak in Germany: a 20-year multicenter study. Hepatology. 2000;32(1):91–6.
Thein HH, et al. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–31.
Datz C, et al. The natural course of hepatitis C virus infection 18 years after an epidemic outbreak of non-A, non-B hepatitis in a plasmapheresis centre. Gut. 1999;44(4):563–7.
Yi Q, Wang PP, Krahn M. Improving the accuracy of long-term prognostic estimates in hepatitis C virus infection. J Viral Hepat. 2004;11(2):166–74.
Kondili LA, et al. Incidence of DAA failure and the clinical impact of retreatment in real-life patients treated in the advanced stage of liver disease: interim evaluations from the PITER network. PLoS One. 2017;12(10):e0185728.
Martini S, et al. The Italian compassionate use of sofosbuvir in HCV patients waitlisted for liver transplantation: A national real-life experience. Liver Int. 2018;38(4):733–41.
Younossi ZM, et al. Treatment of hepatitis C virus leads to economic gains related to reduction in cases of hepatocellular carcinoma and decompensated cirrhosis in Japan. J Viral Hepat. 2018.
Backus LI, et al. Direct-acting antiviral sustained virologic response: impact on mortality in patients without advanced liver disease. Hepatology. 2018.
Poynard T, et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122(5):1303–13.
Maylin S, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology. 2008;135(3):821–9.
Dienstag JL, et al. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54(2):396–405.
Wright M, et al. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess. 2006;10(21):1–113.
Morgan RL, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329–37.
Kondili LA, et al. Modeling cost-effectiveness and health gains of a “universal” versus “prioritized” hepatitis C virus treatment policy in a real-life cohort. Hepatology. 2017;66(6):1814–25.
AASLD Recommendations for testing, managing and treating hepatitis C. 2018. http://hcvguidelines.org. Accessed Nov 2016.
EASL Recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66(1):153–94.
Acknowledgements
The PITER platform has been supported by “Research Project PITER2010” (RF-2010-2315839), awarded to SV. The authors wish to thank the PITER Collaborating group (available at www.progettopiter.it; see also the electronic supplementary material of this article). The model used in this study was provided to the journal’s peer reviewers for their reference when reviewing the manuscript. The members of PITER Collaborating group are: Principal Investigators and Coordinating Group: L. A. Kondili, S. Vella, M. G. Quaranta, S. Rosato, M. E. Tosti, L. E. Weimer, L. Ferrigno, F. D’Angelo, L. Falzano. PITER Investigators: A. Benedetti, L. Schiadà, M. Cucco, A. Giacometti, L. Brescini, S. Castelletti, D. Drenaggi, C. Mazzaro, G. Angarano, M. Milella, A. Di Leo, M. Rendina, A. Contaldo, A. Iannone, F. La Fortezza, M. Rizzi, G. Cologni, L. Bolondi, F. Benevento, I. Serio, P. Andreone, P. Caraceni, V. Guarneri, M. Margotti, G. Simonetti, G. Mazzella, G. Verucchi, V. Donati, P. Mian, G. Rimenti, A. Rossini, G.B. Contessi, F. Castelli, S. Zaltron, A. Spinetti, S. Odolini, G. Leandro, R. Cozzolongo, M. Zappimbulso, M. Russello, R. Benigno, C. Coco, C. Torti, C. Costa, G. Greco, M. Mazzitelli, V. Pisani, L. Cosco, F. Quintieri, M. De Siena, F. Giancotti, J. Vecchiet, K. Falasca, A. Mastroianni, L. Chidichimo, G. Apuzzo, F. G. Foschi, A. C. Dall’Aglio, M. Libanore, D. Segala, L. Sighinolfi, D. Bartolozzi, E. Salomoni, P. Blanc, F. Baragli, B. Del Pin, E. Mariabelli, F. Mazzotta, A. Poggi, A. L. Zignego, M. Monti, F. Madia, A. Xheka, E. M. Cela, T. A. Santantonio, S. Bruno, C. Viscoli, A. I. Alessandrini, C. Curti, A. Di Biagio, L. A. Nicolini, E. Balletto, C. Mastroianni, K. Blerta, D. Prati, L. Raffaele, M. Andreoletti, G. Perboni, P. Costa, L. Manzini, G. Raimondo, R. Filomia, A. Lazzarin, G. Morsica, S. Salpietro, M. Puoti, C. Baiguera, S. Vassalli, M. G. Rumi, S. Labanca, M. Zuin, A. Giorgini, D. Orellana, A. D’Arminio Monforte, A. Debona, S. Solaro, S. Fargion, L. Valenti, G. Periti, S. Pelusi, M. Galli, E. Calvi, L. Milazzo, A. Peri, P. Lampertico, M. Borghi, R. D’Ambrosio, E. Degasperi, M. Vinci, E. Villa, V. Bernabucci, L. Bristot, F. Pereira, L. Chessa, M. C. Pasetto, M. Loi, A. Gori, I. Beretta, V. Pastore, A. Soria, M. Strazzabosco, A. Ciaccio, M. Gemma, G. Borgia, A. Foggia, E. Zappulo, I. Gentile, A. R. Buonomo, N. Abrescia, A. Maddaloni, N. Caporaso, F. Morisco, S. Camera, L. Donnarumma, C. Coppola, D. C. Amoruso, L. Staiano, M. R. Saturnino, N. Coppola, S. Martini, C. Monari, A. Federico, M. Dallio, C. Loguercio, G. B. Gaeta, G. Brancaccio, G. Nardone, C. Sgamato, G. D’Adamo, A. Alberti, M. Gonzo, S. Piovesan, L. Chemello, A. Buggio, L. Cavalletto, F. Barbaro, E. Castelli, A. Floreani, N. Cazzagon, I. Franceschet, F. P. Russo, A. Zanetto, E. Franceschet, S. Madonia, M. Cannizzaro, G. Montalto, A. Licata, A. R. Capitano, A. Craxì, S. Petta, V. Calvaruso, F. Rini, C. Ferrari, E. Negri, A. Orlandini, M. Pesci, R. Bruno, A. Lombardi, V. Zuccaro, R. Gulminetti, A. Asti, M. Villaraggia, M. Mondelli, S. Ludovisi, F. Baldelli, F. Di Candilo, G. Parruti, P. Di Stefano, F. Sozio, M. C. Gizzi, M. R. Brunetto, P. Colombatto, B. Coco, L. Surace, G. Foti, S. Pellicano, G. Fornaciari, S. Schianchi, P. Vignoli, M. Massari, R. Corsini, E. Garlassi, G. Ballardini, M. Andreoni, C. Cerva, M. Angelico, A. Gasbarrini, M. Siciliano, M. De Siena, L. Nosotti, G. Taliani, E. Biliotti, M. Santori, M. Spaziante, F. Tamburini, V. Vullo, G. D’Ettorre, E. N. Cavallari, T. S. Gebremeskel, P. Pavone, R. Cauda, A. Cingolani, S. Lamonica, G. D’Offizi, R. Lionetti, U. Visco Comandini, A. Grieco, F. D’Aversa, A. Picardi, A. De Vincentis, G. Galati, P. Gallo, C. Dell’Unto, A. Aghemo, A. Gatti Comini, M. Persico, M. Masarone, M. Anselmo, P. De Leo, M. Marturano, E. Brunelli, F. Ridolfi, A. M. Schimizzi, M. Ayoubi Khajekini, L. Framarin, G. Di Perri, G. Cariti, L. Boglione, C. Cardellino, L. Marinaro, G. M. Saracco, A. Ciancio, P. Toniutto, G. Alterini, F. Capra, D. Ieluzzi.
Author information
Authors and Affiliations
Consortia
Contributions
AM, FSM, RV, and LAK, designed the study, conducted the analysis and finalized the draft of the manuscript. FSM and SV provided guidance on the methodology, reviewed the results and critically assessed the manuscript. All the authors provided data and/or reviewed the results of the final draft of the manuscript. All authors approved the final version of the manuscript. LAK had full access to all the data used in the study and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
The PITER cohort study protocol was approved by the Ethics Committee of Istituto Superiore di Sanità (Italian National Institute of Public Health) and by the local ethics committees of each clinical centre. Patient data were evaluated via an anonymous analysis, adopting codes generated by the electronic case-report form. Informed consent was obtained from each patient participating in this study.
Conflicts of interest
AM, RV, LAK, SR, FSM and SV have no competing interests to declare regarding the content of this article.
Additional information
The members of PITER collaboration study group are listed in acknowledgements.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marcellusi, A., Viti, R., Kondili, L.A. et al. Economic Consequences of Investing in Anti-HCV Antiviral Treatment from the Italian NHS Perspective: A Real-World-Based Analysis of PITER Data. PharmacoEconomics 37, 255–266 (2019). https://doi.org/10.1007/s40273-018-0733-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-018-0733-3