Abstract
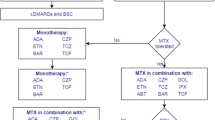
As part of the National Institute for Health and Clinical Excellence (NICE) single technology appraisal (STA) process, the manufacturer of golimumab (Simponi®; Merck Sharp & Dohme, USA) was invited to submit evidence for its clinical and cost effectiveness for the treatment of rheumatoid arthritis (RA) after the failure of previous disease-modifying antirheumatic drugs (DMARDs). The School of Health and Related Research Technology Appraisal Group (ScHARR-TAG) at The University of Sheffield was commissioned to act as the independent Evidence Review Group (ERG). This article provides details of the manufacturer’s initial submission, the ERG’s clarification questions and the ERG report submitted to NICE. The decision made by NICE is provided alongside a brief comment on additional results produced from an additional analysis requested by NICE on behalf of the Committee. The ERG produced a critical review of the evidence for the clinical and cost effectiveness of the technology based on upon the manufacturer’s submission to NICE. The clinical evidence was derived from three randomized controlled trials of golimumab in the treatment of moderate to severe RA: GO-FORWARD and Kay et al. (DMARD-experienced population) and GO-AFTER (tumour necrosis factor [TNF]-α inhibitor-experienced population). The ERG considered that the trials were of reasonable methodological quality and measured a clinically relevant range of outcomes. The trials for golimumab, as well as comparator treatments, were synthesized using mixed-treatment comparison methods for the DMARD-experienced population and an indirect comparison using the Bucher method for the TNF-α inhibitor-experienced population. The trials used were appropriate, although no definitive judgement regarding the comparative efficacy of golimumab with other biologics was possible. The manufacturer provided a DMARD-experienced population model and a TNF-α inhibitor-experienced population model. The models allowed sequences of treatments to be evaluated for each population, although a fully incremental analysis between the use of golimumab following DMARD failure and the use of golimumab following TNF-α inhibitor failure was not possible. Several limitations with the model were identified, and after a request from NICE and suspension of the appraisal, the manufacturer submitted sensitivity analyses with an additional American College of Rheumatology 70 % improvement criteria (ACR70) health state included, using SF-36 data directly from the GO-FORWARD study. The annual rate of the Health Assessment Questionnaire (HAQ) score increase for patients receiving palliative treatment was also changed from 0.09 to 0.06. The further analyses provided highlighted the particular sensitivity of the results to HAQ progression rates and the re-administration frequency for rituximab in the TNF-α inhibitor-experienced population. The Appraisal Committee concluded that golimumab should be recommended in combination with methotrexate as an option for patients with severe active RA who have failed on conventional DMARDs, or who have failed on a TNF-α inhibitor and are contraindicated to or withdrawn from rituximab.
Similar content being viewed by others
References
National Institute for Health and Clinical Excellence. Guide to the single technology appraisal process. London: NICE; 2009.
Sculpher M. Single technology appraisal at the UK National Institute for Health and Clinical Excellence: a source of evidence and analysis for decision making internationally. Pharmacoeconomics. 2010;28(5):347–9.
Rodgers M, Griffin S, Paulden M, et al. Alitretinoin for severe chronic hand eczema: a NICE single technology appraisal. Pharmacoeconomics. 2010;28(5):351–62.
Bagust A, Greenhalgh J, Boland A, et al. Cetuximab for recurrent and/or metastatic squamous cell carcinoma of the head and neck: a NICE single technology appraisal. Pharmacoeconomics. 2010;28(6):439–48.
Stevenson M, Pandor A. Febuxostat for the management of hyperuricaemia in patients with gout: a NICE single technology appraisal. Pharmacoeconomics. 2011;29(2):133–40.
Scotland G, Waugh N, Royle P, et al. Denosumab for the prevention of osteoporotic fractures in post-menopausal women: a NICE single technology appraisal. Pharmacoeconomics. 2011;29(11):951–61.
Dickson R, Bagust A, Boland A, et al. Erlotinib monotherapy for the maintenance treatment of non-small cell lung cancer after previous platinum-containing chemotherapy: a NICE single technology appraisal. Pharmacoeconomics. 2011;29(12):1051–62.
McKenna C, Maund E, Sarowar M, et al. Dronedarone for the treatment of atrial fibrillation: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(1):35–46.
Holmes M, Carroll C, Papaioannou D. Dabigatran etexilate for the prevention of venous thromboembolism in patients undergoing elective hip and knee surgery: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(2):137–46.
Yang H, Craig D, Epstein D, et al. Golimumab for the treatment of psoriatic arthritis: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(4):257–70.
Boyers D, Jia X, Jenkinson D, et al. Eltrombopag for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(6):483–95.
Burch J, Griffin S, McKenna C, et al. Omalizumab for severe persistent asthma in children aged 6–11 years: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(11):991–1004.
Whyte S, Pandor A, Stevenson M. Bevacizumab for metastatic colorectal cancer: a NICE single technology appraisal. Pharmacoeconomics. 2012;30(12):1119–32.
Kilonzo M, Hislop J, Elders A, et al. Pazopanib for the first-line treatment of patients with advanced and/or metastatic renal cell carcinoma: a NICE single technology appraisal. Pharmacoeconomics. 2013;31(1):15–24.
Craig D, Rice S, Paton F, et al. Retigabine for the adjunctive treatment of adults with partial onset seizures in epilepsy with and without secondary generalisation: a NICE single technology appraisal. Pharmacoeconomics. 2013;31(2):101–10.
Spackman E, Rice S, Norman G, et al. Trastuzumab for the treatment of HER2 positive metastatic gastric cancer: a NICE single technology appraisal. Pharmacoeconomics. 2013;31(3):185–94.
Rafia R, Simpson E, Stevenson M, et al. Trabectedin for the treatment of advanced metastatic soft tissue sarcoma: a NICE single technology appraisal. Pharmacoeconomics (In press).
Simpson EL, Fitzgerald P, Evans P, et al. Bivalirudin for the treatment of ST-segment elevation myocardial infarction: a NICE single technology appraisal. Pharmacoeconomics. 2013;. doi:10.1007/s40273-013-0036-7.
Greenhalgh J, Bagust A, Boland A, et al. Rituximab for the first-line maintenance treatment of follicular non-Hodgkin’s lymphoma: a NICE single technology appraisal. Pharmacoeconomics (In press).
Armstrong N, Manuela J, van Asselt T, et al. Golimumab for the treatment of ankylosing spondylitis: a NICE single technology appraisal. Pharmacoeconomics (In press).
Kearns B, Lloyd Jones M, Stevenson M, et al. Cabazitaxel for the second-line treatment of metastatic hormone refractory prostate cancer: a NICE single technology appraisal. Pharmacoeconomics (In press).
Faria R, Spackman E, Burch J, et al. Dabigatran for the prevention of stroke and systemic embolism in atrial fibrillation: a NICE single technology appraisal. PharmacoEconomics (In press).
Symmons D. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology (Oxford). 2002;41(7):793–800.
Cooper NJ. Economic burden of rheumatoid arthritis: a systematic review. Rheumatology (Oxford). 2000;39(1):28–33.
Hyrich KL, Watson KD, Lunt M, et al. Changes in disease characteristics and response rates among patients in the United Kingdom starting anti-tumour necrosis factor therapy for rheumatoid arthritis between 2001 and 2008. Rheumatology (Oxford). 2011;50(1):117–23.
National Collaborating Centre for Chronic Conditions. Rheumatoid arthritis: national clinical guideline for management and treatment in adults. London: NICE; 2009.
Deighton C, O’Mahony R, Tosh J, et al. Management of rheumatoid arthritis: summary of NICE guidance. BMJ. 2009;338:b702.
National Institute for Health and Clinical Excellence. Adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis (TA130). London: NICE; 2007.
National Institute for Health and Clinical Excellence. Certolizumab pegol for the treatment of rheumatoid arthritis (TA186). London: NICE; 2010.
National Institute for Health and Clinical Excellence. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor (TA195). London: NICE; 2010.
National Institute for Health and Clinical Excellence. Abatacept for the treatment of rheumatoid arthritis after the failure of conventional disease-modifying anti-rheumatic drugs (TA234). London: NICE; 2011.
National Institute for Health and Clinical Excellence. Tocilizumab for the treatment of rheumatoid arthritis (TA247). London: NICE; 2012.
National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. London: NICE; 2008.
Jackson R, Tosh J, Davis S, et al. Golimumab for the treatment of rheumatoid arthritis after failure of previous disease-modifying antirheumatic drugs: a single technology appraisal. Sheffield: ScHARR; 2010.
Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor alpha given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD study. Ann Rheum Dis. 2009;68(6):789–96.
Kay J, Matteson EL, Dasgupta B, et al. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58(4):964–75.
Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374(9685):210–21.
Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363(9410):675–81.
Hurst NP, Kind, P, Ruta D, Hunter M, Stubbings, A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Rheumatology. 1997;36:551.
National Institute for Health and Clinical Excellence. Rheumatoid arthritis (after failure of previous antirheumatic drugs): golimumab—appraisal consultation document. London: NICE; 2010. http://guidance.nice.org.uk/TA/Wave19/53/Consultation/DraftGuidance. Accessed 26 Mar 2013.
National Institute for Health and Clinical Excellence. Final appraisal determination: golimumab for the treatment of rheumatoid arthritis after the failure of previous disease-modifying antirheumatic drugs. London: NICE; 2011.
Acknowledgments
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme (project number 08/118/01 STA) and will be published as part of a compendium of ERG articles in Health Technology Assessment. See the HTA programme website (http://www.hta.ac.uk) for further project information. This summary of the ERG report was compiled after the AC’s review and incorporates additional information and comment from the authors on the STA process and iterations of the NICE guidance not covered by the HTA report. This summary has not been externally peer reviewed by PharmacoEconomics. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of NICE or the Department of Health. The authors have no conflicts of interest that are directly relevant to the content of this summary.
Author Contributions
JT is the guarantor for the content of this paper. RA and SD co-authored this paper. All authors (JT, RA, SD, MS and JWS) contributed to the writing of the paper and all have reviewed the final version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tosh, J., Archer, R., Davis, S. et al. Golimumab for the Treatment of Rheumatoid Arthritis After the Failure of Previous Disease-Modifying Antirheumatic Drugs: A NICE Single Technology Appraisal. PharmacoEconomics 31, 653–661 (2013). https://doi.org/10.1007/s40273-013-0052-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-013-0052-7