Abstract
Background
Lipodystrophy comprises a group of conditions characterized by loss of functional adipose tissue, resulting in severe metabolic complications and a complex range of symptoms.
Objective
This study sought to gain a holistic understanding of the impact of congenital or non-human immunodeficiency virus acquired lipodystrophies on the quality of life of patients and their caregivers and to capture the impact of lipodystrophy on quality of life using a standard instrument.
Methods
Ten patients with lipodystrophies and five caregivers from the USA and UK were recruited through convenience sampling and interviewed using a semi-structured questionnaire containing open-ended questions about disease symptoms and attributes and numerical rating scales to prompt discussion of symptom prevalence and impact. After the interview, participants filled out the 36-Item Short Form (SF-36) survey instrument. Conventional conceptual content analysis methods were used to analyze the anonymized transcripts.
Results
Four concepts were developed: diagnostic journey and symptom management, burden of disease, healthcare resource utilization, and support and advocacy. Participants described lengthy diagnostic journeys and frequent interactions with healthcare systems. Many participants became experts on lipodystrophy through the diagnostic journey and described difficulties accessing effective treatment, even after diagnosis. Both patients and caregivers emphasized the ongoing burden of living with lipodystrophy and the accompanying sense of isolation. Participants turned to disease-specific support groups to cope, engaging in knowledge sharing with other patients and caregivers and developing friendships based on shared experiences. Ten participants completed the SF-36, with a mean (standard deviation) SF-36 score of 0.6 (0.2).
Conclusions
Currently, there are no qualitative studies that describe the experiences of patients with lipodystrophy and their caregivers. While additional research is needed, educational work like this study is a promising first step that could lead to early diagnosis and access to treatment and support.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ten patients with lipodystrophies and five caregivers from the USA and UK, together representing the experiences of 12 patients, conveyed the challenges and isolation they experienced as they navigated the diagnostic journey, interacted with healthcare providers, sought appropriate treatment, and learned how to manage the wide range of symptoms associated with congenital or non-human immunodeficiency virus acquired lipodystrophies. |
To cope with the significant burden of daily symptom management and the acute sense of isolation, many participants turned to disease-specific patient support groups and expert clinical centers for emotional support, care coordination resources, and disease management best practices. |
It is important to increase disease awareness to ensure an early diagnosis of lipodystrophy syndromes and a timely initiation of appropriate treatment. |
1 Introduction
Lipodystrophy comprises a group of inherited or acquired rare and complex conditions characterized by loss of functional adipose tissue, which can result in severe metabolic complications [1,2,3,4]. As a result, fat accumulates in non-adipose tissues, leading to metabolic and cosmetic abnormalities such as hyperglycemia, severe insulin resistance, hypertriglyceridemia, and a muscular physique. These abnormalities are often associated with the development of acute pancreatitis, hepatic steatosis, cirrhosis, cardiovascular disease, end-stage renal disease, and other complications. Patients with lipodystrophy often have reduced levels of leptin, a hormone produced by adipose tissue that regulates several metabolic processes [4]. Deficient levels of leptin have been associated with the manifestation of various metabolic abnormalities [4]. Leptin replacement therapy has been shown to improve glycemic control and decrease triglyceride and hemoglobin A1c levels, markers of lipodystrophy severity [5, 6].
1.1 Impact of Disease on Patients
A systematic literature review estimated that there are 1.3–4.7 cases of non-human immunodeficiency virus (non-HIV)-associated lipodystrophy syndromes per million people [7]. Because of limited clinical expertise in rare diseases, it is common for patients or their caregivers to become actively engaged in seeking to understand their disease state [8,9,10,11,12]. The diagnostic journey can be arduous for these patients, who may be initially misdiagnosed or receive inappropriate medical interventions [13].
At present, there is a paucity of literature regarding lipodystrophy-associated patient-oriented outcomes. Pilot data from LD Lync, a prospective data registry, suggest that lipodystrophy syndromes present a significant burden to patients [14]. The European Organisation for Rare Diseases reports that educational attainment and work productivity loss are largely due to absenteeism in patients and that caregivers experience a substantial time burden associated with daily care and coordination [9]. Given the complex and heterogeneous nature of lipodystrophy, it is necessary to characterize the multifaceted experience of how patients and their caregivers confront daily challenges. The objectives of this study were to capture the experiences of patients with lipodystrophies and their caregivers and understand the impact the disease has on quality of life and healthcare resource utilization. Specifically, we sought to (1) gain insight into the impact of lipodystrophy on the lives of patients and caregivers beyond what can be captured by directly inquiring about symptoms of lipodystrophy and associated disease attributes, (2) establish which symptoms of lipodystrophy and associated disease attributes resonate the most with patients and caregivers in terms of disease burden and related challenges, and (3) gain an initial perspective of the patient and caregiver assessment of the impact of lipodystrophy on quality of life by using a standard instrument.
2 Methods
A subset of the authors (AG, KC, ET) was involved in a larger evidence generation effort on lipodystrophy and leptin replacement therapy. The list of symptoms of lipodystrophy and associated disease attributes that were included in the interview guide used in this study was originally generated to inform a discrete choice experiment designed to quantify the quality-of-life consequences of lipodystrophy disease attributes recorded in medical chart data of a cohort of 112 patients with generalized lipodystrophy (GL) and partial lipodystrophy (PL) who were treated with leptin replacement therapy at the US National Institutes of Health (NIH) [15]. The list of symptoms of lipodystrophy and associated disease attributes was reviewed and confirmed by NIH clinical experts who supported the discrete choice experiment study. The interview guide for this study was developed based on this list and the data generated through the discrete choice experiment. The final interview guide contained qualitative open-ended questions about the symptoms of lipodystrophy and associated disease attributes, as well as numerical rating scales to prompt discussion of symptom prevalence and its impact on quality of life and healthcare resource utilization.
A convenience sample of patients with lipodystrophy and caregivers of patients with lipodystrophy were interviewed by an independent researcher using the semi-structured interview guide. At the end of the interview, participants were asked to fill out the Optum™ SF‐36v2® Health 36‐Item Short Form (SF‐36) survey instrument. Once the interviews were transcribed and anonymized, conventional conceptual content analysis methods were used to analyze the qualitative data. Although the SF‐36 survey instrument is commonly used to measure health‐related quality of life across various diseases, it has not been validated for use among patients with lipodystrophy and thus our intent was to gather preliminary data to further document the impact of lipodystrophy on patients’ quality of life.
2.1 Study Participants and Recruitment
The data source was 15 patient/caregiver interviews of approximately 60 minutes in length conducted between August 2017 and February 2018. Adult patients with lipodystrophy and caregivers of patients with lipodystrophy were recruited through convenience sampling, using patient groups in the USA and UK. Given that lipodystrophy is a rare disorder, we did not set out with a predetermined sample size or plan to limit the sample size upon reaching theoretical saturation. Instead, we interviewed all patients and caregivers who were interested in the study and available to participate. In the UK, members of Lipodystrophy UK were invited to participate in this study via e-mail. In the USA, Aegerion Pharmaceuticals Inc. (study sponsor) invited patients with lipodystrophy who were participants in their patient support programs and who were registered to attend a research conference in Boston, MA to participate in this study via e-mail. Although patients aged < 18 years were not eligible to participate in this study, caregivers of pediatric patients were eligible to participate.
2.2 Data Collection Procedures
A male researcher (CF, PhD) with extensive experience in qualitative data collection, focus group moderation, and health services research who was a colleague of the authors (AG, KC, AW, ET) at the time of the study conducted the interviews with each of the participants. Patients from the UK were interviewed via telephone and patients in the USA were interviewed in person in a private conference room in Boston, MA, unless they stated a preference to be interviewed via telephone. Prior to the interview, the researcher informed the participants that the interview would be audio recorded and transcribed to inform research to support the understanding of lipodystrophy and its burden and that the research would help with the development of new therapies to address the disease. The researcher also informed participants that, while he was conducting the study on behalf of the study sponsor, any information collected would be treated as confidential and blinded during the transcription process, and that all study results would be reported as aggregate to the study sponsor and in the case of public dissemination. Interviews only began after each participant verbally communicated to the researcher his/her consent to participate in the study and be recorded. Further, data collected during the interview were only used for analysis if consent was given after completion of the interview. Only the researcher and the participant(s) were on the telephone line (for UK participants) and conference room (for US participants) during the interview and field notes were written after each interview ended. No repeat interviews were conducted and transcripts were not returned to participants.
The interview guide, which contained open-ended questions and numerical rating scale exercises to prompt discussion, recorded key patient demographics, diagnosis and disease history, treatment history, healthcare resource utilization, and the presence and impact of symptoms of lipodystrophy and disease-specific attributes (e.g., organ damage, ability to perform activities of daily living, mental health disorders, metabolic complications, and neuropathy) on both patients’ and their caregivers’ quality of life via open-ended questions and allowed the researcher to probe in reaction to participants’ comments. Although fatigue and prior experience with leptin therapy were not included in the interview guide, multiple participants volunteered information on these matters. Therefore, we included these data in the analyses.
Participants were not provided with the interview materials beforehand. During the interview, the researcher shared with the participants a three-column table listing symptoms of lipodystrophy and disease-specific attributes in the first column and a prompt to assign a numerical value between 0 (no impact) and 10 (highest impact) to each of the symptoms and attributes in the second column. In the third column, patients were asked to provide a rating between 0 (no impact) and 10 (highest impact) for future concern, regardless of whether they had experienced the symptoms and disease attributes. The intent of this rating exercise was not to objectively capture the impact of the symptoms of lipodystrophy and associated disease attributes to patients’ daily life and future concerns. Rather, we anticipated that each participant would engage with the rating scale differently and used it as a tool to prompt discussion. At the end of the interview, the researcher shared with each participant the SF‐36 survey instrument. As per SF-36 guidelines, caregivers were asked to complete the questionnaire on behalf of patients who were unable to complete the survey themselves. Caregivers were encouraged to obtain input from the patient when possible. Although the instrument has not been validated in patients with lipodystrophy, our intent was to gather preliminary data to further document the impact of lipodystrophy on patients’ quality of life.
2.3 Analyses
Once the interviews were transcribed and anonymized, conventional conceptual content analysis methods were used to analyze the qualitative data [16, 17]. This approach was chosen as it is well suited when existing theory or published literature on a phenomenon is limited [16]. The data analysis process started with three researchers (AG, AW, KC) reading all data repeatedly to obtain a sense of the whole. Then, two of the researchers (AG, AW) read the data at the theme level to derive codes by first highlighting the exact sentences from the text that appeared to capture key thoughts or concepts and then making notes of their first impressions, thoughts, and initial analyses. After open coding four transcripts, labels for codes emerged that were reflective of more than one key thought. These codes were the building blocks for the initial coding scheme. Codes were then sorted into clusters based on how different codes were related and linked. Depending on the relationship between clusters, researchers combined and organized these into a smaller number of higher level categories with the help of a coding tree diagram (see Electronic Supplementary Material [ESM]). Next, a codebook that contained the definitions for each category and code was developed. The two researchers (AG, AW) then applied the codes to seven transcripts (representing the experience of three caregivers and four patients) and reached approximately 95% inter-coder reliability upon adjudication. Subsequently, a senior researcher (KC) not involved in the initial coding tested the refined codebook by coding a transcript. Once again, the codebook was refined to address discrepancies. One of the original researchers (AG) then applied this latest version of the codebook to another transcript. Cohen’s Kappa was calculated for each individual code. Only codes with a Cohen’s Kappa above 0.90 were retained. The researchers (AG, AW) then coded the remaining transcripts (and recoded the original transcripts) using the final version of the codebook. Once all transcripts had been coded, the researchers (AG, AW, KC) analyzed the results of the coding process to capture the thematic concepts within the data. All data management and analysis was conducted using Microsoft Word 2016.
Because the intent of the numerical rating exercise was to prompt discussion, only basic descriptive statistics were conducted on the quantitative data. The total number of patients who experienced each symptom and disease-related attribute were calculated by counting the number of patients for whom a given attribute is applicable and with whom a given attribute was discussed. Mean impact ratings were also calculated using data from the number of total patients for whom a given attribute was applicable and with whom a given attribute was discussed.
The data collected via the SF-36 were analyzed independently from the data collected through the interview guide. The main outcome measure derived from the SF-36 patient-reported outcomes instrument is a SF-6D score. The SF-6D score estimates a preference-based single index measure for health from these data using general population values. The lower the score the greater the perceived disability, i.e., a score of zero is equivalent to maximum disability and a score of 100 is equivalent to no disability. The SF-36 surveys were scored using Optum proprietary software. The development of the study design and interview materials for this study takes into account the 32 items on the Consolidated Criteria for Reporting Qualitative Research checklist (see ESM) [18].
3 Results
3.1 Participant Characteristics
Fifteen individuals (ten patients and five caregivers) consented to participating in recorded interviews conducted between August 2017 and February 2018. None of the patients or caregivers who agreed to participate retracted consent during or after the interview process. Table 1 summarizes the demographic make-up of the participants, with 15 participants reflecting the experience of 12 individual patients with GL (n = 4) or PL (n = 8). These participants comprised ten adult patients who were interviewed directly, three caregivers of two pediatric patients who described their children’s experience, and two additional caregivers who joined their adult child for the interview. Nine of the ten patients and three of the five caregivers who participated in this study were female.
3.2 Symptoms and Disease Attributes
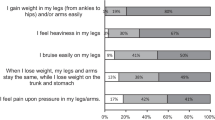
All patients experienced symptoms as children, and six patients with GL and PL experienced symptoms as newborns (Table 2). The median time between age at first symptom and diagnosis was < 1 year but the range was < 1 year to 30 years. Of patients diagnosed within 1 year (N = 6), three had GL and three had PL, suggesting no difference between lipodystrophy type and time to diagnosis. However, among patients with a time to diagnosis of > 10 years, four had PL and one had GL, suggesting patients with PL may have a longer and more complex path to diagnosis. The patients experienced a wide range of lipodystrophy-related symptoms and disease attributes. Those reported by more than 75% of the participants included hyperphagia (92%), uncontrolled blood glucose (92%), inability to perform usual activities (83%), inability to attend work/school (83%), impaired mobility (83%), altered physical appearance (83%), anxiety (82%), neuropathy (82%), depression (80%), pain/discomfort (75%), and uncontrolled triglyceride levels (75%) (Fig 1). Those reported by < 50% of participants are advanced bone age, heart damage, pancreatitis, and amputations, which may reflect the current age of the participants: mean 31.8 years, median 33.5 years, and range 1.5–57 years. Nine of the ten patients and all five caregivers interviewed described a significant negative impact of lipodystrophy on their life. Patients rated several disease-related attributes as having a mean impact rating ≥ 5 on this study’s scale of 0 (no impact) to 10 (high impact): fatigue (8.5), hyperphagia (6.9), inability to attend work/school (6.3), inability to perform usual activities (5.9), anxiety (5.6), depression (5.2), and physical appearance (5.0) (Fig. 2). For caregivers, loss of work opportunities, decreased productivity and/or income, the need to accommodate their schedule and meals for the entire family based on the patient, and the distress and stigma caused by social interactions and the public’s unfamiliarity with the disease add to the burden of caring for a child with lipodystrophy. The SF‐36 survey instrument was completed (self-administered or via interview) by ten participants with a mean (standard deviation) SF-6D score of 0.6 (0.2).
Number of patients who experienced symptoms and disease-related attributes, n = 12 for all attributes except anxiety (n = 11), depression (n = 10), neuropathy (n = 11), reproductive functioning (n = 9), and advanced bone age (n = 11). Totals were calculated by counting the number patients for whom a given attribute is applicable and with whom a given attribute was discussed. Fatigue was not included in the original discussion guide but was raised by respondents unprompted
Mean impact rating of reported disease-related attributes across all patients, n = 12 for all attributes except anxiety (n = 11), depression (n = 10), neuropathy (n = 11), reproductive functioning (n = 9), and advanced bone age (n = 11). To capture the impact of symptoms and attributes to patients’ daily life, respondents were asked to assign a numerical value between 0 (no impact) and 10 (highest impact) to each of the symptoms and attributes. Means were calculated from the number of total patients for whom a given attribute was applicable and with whom a given attribute was discussed. Fatigue was not included in the original discussion guide but was raised by respondents unprompted
3.3 Concepts
Four concepts were developed (Table 3): diagnostic journey and symptom management; burden of disease; healthcare resource utilization; and support and advocacy. Participant quotations are presented to illustrate the findings. However, quotations have not been identified with a participant number to protect anonymity.
3.3.1 Diagnostic Journey and Symptom Management
3.3.1.1 Diagnostic Journey
Many participants reported that clinicians were not familiar with the disease and were unable to make an accurate initial diagnosis. Symptoms appeared during childhood for both patients with GL and PL; three out of four patients with GL experienced symptoms as newborns. Time between age at first symptom and diagnosis ranged from < 1 year to 30 years (Table 2).
Participants reported having to convince providers to go beyond treating specific symptoms (e.g., uncontrolled triglyceride levels). Several participants expressed frustration with the lengthy diagnostic journey but great appreciation towards the clinicians who eventually made the diagnosis. A participant from the UK with PL was not diagnosed until she was 25 years of age, although she was treated for symptoms such as cardiomyopathy from her teenage years.
“… I used to go to the doctor saying, ‘I feel ill. This is wrong, this is wrong.’ And they say to me it’s all in my head and there’s nothing wrong with me … But then I’d actually seen a good endocrinologist and I told him my concerns and he did about ten sets of tests because he couldn’t understand why I’ve got type 2 diabetes and I was like thin, and then the final word came back that I’ve got familial partial lipodystrophy.”
Once lipodystrophy syndromes were diagnosed, or as a caregiver said, “once we knew to look”, patients transitioned to clinicians who were more familiar with the disease and received guidance and treatment, to manage symptoms. Overall, participants with a lengthy diagnostic journey felt like they should not have had to advocate as much as they had, but also expressed feeling validated and less “neurotic” once the diagnosis was received.
3.3.1.2 Symptom Management
Most participants experienced a phase following diagnosis when providers used an empirical approach to achieve personalized treatment. For a number of participants, this phase evoked a sense of mistrust. One patient described feeling like “a test dummy” and was reluctant to attend all check-ups. One caregiver even ignored nutritionists’ advice and developed her own dietary regimen for her daughter. Once diagnosed with lipodystrophy, participants also found it challenging to gain access to therapy.
All but one participant indicated that it was difficult and resource intensive (in terms of time, energy, or money) to manage their symptoms owing to complex logistics related to preparing meals, taking medications as indicated, attending related medical appointments, and balancing work and time with loved ones with the need to rest because of chronic fatigue. The unpredictability of symptom onset, in particular pain and fatigue, added to the burden.
3.3.2 Burden of Disease
3.3.2.1 Clinical Burden of Disease
Lipodystrophy syndromes impact patients’ physical health (from organ damage to muscle spasms), reproductive health, sexual health, mental health, sleep, mobility, and vitality. Patients and caregivers were particularly concerned about the risk of organ damage and its progression. Pain associated with advanced bone age, neuropathy, irregular menstrual cycles, and muscle spasms was identified as a barrier to executing common tasks (e.g., talking, typing, doing household chores, and running errands). Patients also singled out hyperphagia as a source of significant physical discomfort and emotional distress.
“… sometimes since we’re so hungry, we’ll binge, binge, binge, binge, and then like make ourselves sick and then don’t want to eat anything at all … but we have to keep eating, if that makes any sense. So yeah, it’s terrible … starving all the time.”
The impact of lipodystrophies on reproductive health was a source of anxiety and concern for female patients and their caregivers. Several patients with children conveyed that they discourage other patients from trying to get pregnant because symptoms become unmanageable during this time. Caregivers also expressed concern about their daughters experiencing pregnancy; none wanted their daughters to go through pregnancy.
“Yes, I had a miscarriage last year … They told me I was high risk in the first place, and I had to stop working. It only lasted seven and a half weeks before it was gone … talked to me yesterday about just all of the risk of pregnancy, it’s a life and death type of thing.”
Difficulty managing symptoms, acknowledgment of likely disease progression, and others’ reaction to their appearance seemed to take a toll on patients’ mental health. Experiences of depression and anxiety, often managed by pharmaceutical treatment, were also common among patients. Two of the ten patients conveyed experiencing suicidal thoughts because of the burden of lipodystrophy syndrome.
“I felt like I was doing so much and nothing was helping and I just kind of hit that point, I would say kind of rock bottom to where I just didn’t care anymore. I didn’t care if my medicine was working or if it wasn’t working. I just kind of got the attitude where I was—and I would even tell my family members as well. I would say ‘If it’s time for me to go, everyone dies when they die.’”
3.3.2.2 Humanistic Burden of Disease
All but one patient described symptoms as barriers to developing or maintaining interpersonal and professional relationships. Planning their schedules around availability of food, having dietary restrictions, and needing to “allocate energy” across interactions and chores were cited as causes for strained relationships.
“My fatigue is great enough where I really limit activities. So if I spend time with my kids on Saturday, I’m going to have to rest on Sunday. So, I mean, it’s impacted everything. It impacted my marriage. Now I can say, retrospectively, for sure, it impacted my marriage.”
Physical appearance was frequently mentioned as a barrier to new relationships. One patient expressed feeling offended by how others approached her about her muscular appearance and said she preferred to keep to herself to avoid comments. Caregivers expressed concern about the impact that physical appearance would have on their daughters’ self-esteem, and their ability to find a romantic partner.
“You’ll get stares in public you know, ‘Who is that,’ or people talking about you, you know, like, ‘Look at that man over there,’ or, ‘Why your girlfriend so strong,’ you know ... that kind of gets to you sometime because people are not used to seeing strong women, as strong as we are ...”
It was apparent patients had developed coping strategies to manage symptoms and consequences related to lipodystrophy, as also seen in other rare diseases [19]. The need for such coping strategies was illustrated by one patient who described her hyperphagia as a less severe form of “addiction than alcohol or drug abuse”. The most common coping strategy employed was compartmentalization of burden, defined in this study as instances in which a patient distinguishes between how lipodystrophy impacts the self as a whole vs different aspects of his/her life.
“I love my physique … [but] because it makes other people uncomfortable, it makes me uncomfortable. And I think that’s what—it’s the bullying—it’s all psychological for me. Like I don’t think it’s more so my condition that I’m sick, it’s psychological, because it’s like, “Okay, I love myself.” And it’s like in my house I feel beautiful… But then when I go out to the world, where everybody else is different, it kind of makes me feel like an outcast. So that makes me feel uncomfortable. But no, I’m not ashamed.”
Familial responsibility also emerged. Patients expressed anxiety, guilt, and feeling responsible for the genetic testing status and results of their blood relatives and expressed distress when a family member refuses to get tested. Patients of childbearing age expressed great concern about passing on lipodystrophy to future children.
3.3.2.3 Economic Burden of Disease
Patients discussed high rates of school/work absenteeism due to lipodystrophy effects, including chronic pain, hyperphagia, and fatigue. Patients also identified that a lack of access to higher education resulted in limited professional opportunities.
“So I went to college and because I got so bullied and I just didn’t want to go in because I didn’t want to get bullied. So I just stopped going, but I know if that didn’t happen I would’ve stayed in college and I would have achieved it and I would be where I want to be by now.”
3.3.2.4 Burden of Disease on Caregivers
The impact of lipodystrophy on caregivers and patients’ families included work loss resulting in reduced household income, social stigma, and challenges navigating healthcare systems. Two of three female caregivers discontinued or limited employment to provide care.
“I had to quit work, she kept getting so sick, it was just a matter of time before they cut me loose anyway. But I had to leave work and never go back, I had to leave school, I mean I have 18 years of college education and I’m a stay at home mom for a rare disease patient.”
Caregivers expressed caregiver guilt and anxiety owing to their perceived lack of preparedness to care for a patient with lipodystrophy. One of five caregivers conveyed experiencing suicidal thoughts.
“I think I was so crushed when she was diagnosed, so that had a huge impact on my mental health as well and so that was, like, a huge hit, like a huge truck … ”
A common concern observed in other rare diseases is that siblings of a child affected by a rare disease often feel neglected [9]. In lipodystrophy, because of patients’ special dietary needs and hyperphagia, significant accommodations to modify meals for the entire family were reported. One caregiver did not allow her other children to eat sweets in front of the patient as she was worried the young patient would “realize what she has been missing out on.” All caregivers were concerned about how their children would manage symptoms on their own.
Caregivers were also concerned about social interactions their children were being deprived of as they did not feel comfortable allowing their children around others because of weakened immune systems. They also expressed apprehension at the lack of educational institutions that would be able to accommodate their children’s special needs.
3.3.3 Healthcare Resource Utilization
Patients and caregivers described high healthcare resource utilization. One infant was diagnosed through newborn screenings and, after lipodystrophy syndromes were diagnosed, she was hospitalized for 11 days. Then she had biweekly check-ins. Once she started leptin therapy, her check-ins became monthly and now she only attends an annual appointment at the NIH. Another caregiver, who had to advocate for a diagnosis, described high-resource utilization throughout her daughter’s diagnostic journey. Before diagnosis, the caregiver reported multiple visits to the pediatrician and an emergency room visit. In another case, the caregiver felt that local clinicians lacked the expertise to treat her daughter, so she sought treatment elsewhere before referral to the NIH. Since then, the NIH has been the primary source of care and symptom management for this patient. Overall, this caregiver indicated that healthcare resource utilization had decreased following diagnosis and initiation of leptin therapy. [While discussing the topic of healthcare resource utilization, nine of 12 participants volunteered unprompted information about the impact of leptin therapy in addition to current standard of care (Table 2).] Mental health treatment was also described:
“I was seeing a counselor. I wasn’t on medication, but I was meeting a counselor every week for a while and then it got better, so I see her whenever I need to see her, maybe like once a month …”
Prior to diagnosis, patients described multiple procedures and hospitalizations for “bizarre, unexplained issues”. One patient reported being hospitalized at least four times for pancreatitis and another patient described taking approximately 15 different medications to manage her symptoms. Of the nine patients who were treated with leptin therapy, seven expressed improved symptom management, and three indicated fewer hospital visits. Four patients also mentioned reduced usage of insulin to control their blood glucose levels.
“Prior to leptin, I was in various appointment systems several times a week. But once I was diagnosed with lipodystrophy and then got the leptin because everything started to work properly, I mean, I just go once every three to six months now to the various consultants I see.”
Patients who mentioned experiencing mental health disorders reported treatment with behavioral therapy and pharmaceutical drugs. The most common barriers to treatment were related to participants not trusting their providers because they felt like the providers were experimenting on them. Some patients described not seeking treatment for pain and mental health issues because of previous experiences of emergency department clinicians chastising or ridiculing them and not taking their complaints seriously. One patient from the UK characterized a lack of coverage through the National Health Service as the main barrier to addressing her emotional distress. She described struggling to find group therapy to help her cope with the impact of her altered appearance. Other patients identified the use of pharmaceuticals to address mental health disorders, including depression and anxiety.
3.3.4 Support and Advocacy
Both patients and caregivers reported receiving emotional and care-coordination support from immediate caregivers, members of nuclear family, patient groups, and clinical providers who have significant expertise and experience with lipodystrophy syndromes. In particular, caregivers and patients engaged in patient support groups reported benefiting from patient-to-patient, caregiver-to-caregiver, and (in one instance), patient-to-caregiver knowledge sharing. Information shared consisted of best practices and tried-and-true methods to cope with hyperphagia, administer medication, and navigate the healthcare system. Occasionally, older patients would provide parenting and caregiving advice to parents.
“And so when the symposium came, I had found a mother who has a daughter with CGL … So we ended up in NIH, and that’s where she’s being treated at ... and that’s her long-term clinical trial there, for the medication she’s on … And I was like, ‘Why did you ask me to do that?’ She goes, ‘I wanted you to learn. I wanted you to know, but I wanted you to know from a mother’s perspective.’ And now, in the community, I’m the mother that takes care of the mothers.”
A byproduct of seeking support and benefiting from interactions with patient groups is motivation to become involved in advocacy efforts. Raising awareness, providing peer support, and sharing best practices with other patients and caregivers were among the motivating factors described.
4 Discussion
This is the first study to explore the qualitative experiences and perspectives of patients with GL and PL and their caregivers. Patients and caregivers described similar experiences in terms of the diagnostic journey, interactions with healthcare systems, and the process of learning how to manage their symptoms. Both patients and caregivers characterized the burden of living with lipodystrophy and accompanying isolation. To cope, participants turned to disease-specific support groups and engaged in knowledge sharing with other patients and caregivers, developing friendships based on their shared experiences.
Often, increased time to diagnosis and treatment was driven by delays in seeing physicians familiar with the disease and/or specialized treatment centers; many participants became experts on lipodystrophy through their diagnostic journey. Even after diagnosis, however, participants described difficulties accessing effective treatment. This is a common experience for patients with rare disorders [8, 10,11,12]. While not an objective of this research, nine of 12 participants volunteered unprompted information about the impact of leptin therapy in addition to current standard of care (Table 2). Seven patients reported improvement in symptoms following initiation of leptin therapy. Of these, six participants reported substantial improvements in hyperphagia, four in blood glucose levels, and two in triglyceride levels, following initiation of leptin therapy. These findings are not unique. In a retrospective analysis of patients with GL or PL who were treated with leptin therapy in an early access program, all patients at baseline had at least one form of organ impairment (i.e., liver impairment, cardiovascular damage, kidney impairment, and pancreatitis), 93% had diabetes mellitus, and 95% of patients with GL and 71% of patients with PL had high triglyceride levels and 78% of patients with GL and 68% of patients with PL had high blood glucose levels [20]. By month 12, the mean decrease in triglyceride levels was 32.1% among patients with GL and 37.4% among patients with PL and the mean percentage point decrease in blood sugar levels was 2.2 among patients with GL and 0.9 among patients with PL.
Managing varied and complex lipodystrophy syndromes requires a significant amount of effort from both patients and caregivers. Patients frequently reported uncontrolled blood glucose, hyperphagia, abnormal physical appearance, impaired mobility, and reduced ability to perform work, attend school, and perform usual daily activities. Nearly all (11/12) patients experienced hyperphagia and the symptom was reported to be acute, its presence chronic, and the feeling of hunger quite different from that of individuals without lipodystrophy. Additionally, chronic fatigue, though not included a priori in the questionnaire, was reported as having a substantial impact. The prevalence of lipodystrophy symptoms and associated disease attributes among our study participants is not surprising. An international chart review study assessing the natural history of non-HIV-related GL and PL in patients who have never received leptin or other lipodystrophy-specific therapies identified diabetes/insulin resistance in 58.3% of patients, liver abnormalities in 71.7%, kidney abnormalities in 40.4%, heart abnormalities in 30.4%, and pancreatitis in 13% of patients [21].
Overall, patients with lipodystrophy seem to experience reduced quality of life (mean SF-6D score of 0.6 compared to 1 for a person in perfect health). Although the SF‐36 survey instrument is commonly used to measure health‐related quality of life across various diseases, it has not been validated for use among patients with lipodystrophy. For context, a study examining the health-related quality of life of individuals aged ≥ 65 years who had been diagnosed with relatively rare cancers (bladder, melanoma, uterus, non-Hodgkin lymphoma, kidney, cervix, oral cavity and pharynx, thyroid, ovary, upper gastrointestinal, chronic leukemia, multiple myeloma, and pancreas) using data from the Surveillance, Epidemiology, and End Results national cancer registry system linked to the Medicare Health Outcomes Survey found that the mean SF-6D score (95% confidence interval) among cancer survivors ranged between 0.63 (0.62–0.65) and 0.71 (0.71–0.72) compared to a mean SF-6D score (95% confidence interval) of 0.73 (0.73–0.73) among individuals aged ≥ 65 years with no history of cancer [22].
Among the patients in this study, 82% reported experiencing anxiety, 80% depression, and 67% fatigue. While leptin deficiency may influence symptoms of depression via its role in neural plasticity, many participants also described anxiety and depression as stemming from the phenotypic expression of symptoms [23]. Patients in this study also experienced disease-related limitations in terms of educational and professional development, which added to the burden of living with this disease.
Patients with lipodystrophies report significant barriers in receiving a diagnosis, interacting with multiple clinicians, and experiencing skepticism or inappropriate treatment. Moreover, patients and their caregivers often resort to resource-intensive methods that have variable success to manage lipodystrophy-associated symptoms. Consequently, healthcare professionals should consider patient and caregiver perspectives when providing holistic care to patients with lipodystrophies or other rare diseases. Further investigation of patients’ experiences with lipodystrophy treatments (e.g., metreleptin, reported among 9/12 patients) could identify care regimens that lead to improved patient-reported outcomes.
Study participants’ experiences with symptom management, burden of disease, healthcare resource utilization, and patient groups may be similar to those of other patients with rare disorders that require complex care management [9, 19, 24]. The experiences of patients with lipodystrophy and their caregivers reported in this study may be most transferable to studies of other patients who have non-HIV-related lipodystrophies and their caregivers and reside in countries with healthcare systems similar to those in the USA and UK. This study may also be relevant to patients who have other chronic rare metabolic disorders in which their daily lives are severely affected and reside in countries with healthcare systems similar to those in the USA and UK.
4.1 Limitations
The present research also has several limitations. Only adults from the USA and UK (mostly female) were interviewed. Pediatric experiences and perspectives are presented through the lens of caregivers. Our interview guide did not include a structured question regarding treatment with leptin therapy. Therefore, data associated with this treatment are only available from patients who volunteered their experiences with this treatment. Additionally, the interview guide was not systematically pilot tested prior to the interviews and the study findings were not shared with participants for respondent validation. Given that lipodystrophy is a rare disease, future qualitative studies seeking to understand patient and caregiver experiences may be able to reach and include more participants across a greater range of geographical regions by leveraging local and global patient groups, as well as registries maintained by medical institutions and pharmaceutical companies.
5 Conclusions
This is the first study to explore the qualitative experiences and perspectives of patients with lipodystrophy and their caregivers. The patients and caregivers who participated in this qualitative study shared their experience of living with lipodystrophy, including key symptoms and the burden associated with this severe disease, as well as the indirect impacts of living with lipodystrophy, such as the lack of social interaction and limited career opportunities. In particular, they conveyed the challenges and isolation as they navigated the diagnostic journey, interacted with healthcare providers, sought appropriate treatment, and learned how to manage varied symptoms.
An important aspect of this study is the exposition of the complexity of the disease attributes and the difficult diagnostic journey. Some of the symptoms discussed, such as anxiety, depression, chronic pain, impact on mobility, and fatigue, are not yet recognized as being associated with lipodystrophy syndrome. While many participants eventually found disease-specific support groups and expert centers (e.g., the clinical center at the National Institute of Diabetes and Digestive and Kidney Diseases at the NIH), it is important to continue to increase disease awareness among the clinical community to ensure an early diagnosis and a timely initiation of appropriate treatment. While additional research is needed to better characterize initial symptoms that could lead to an early diagnosis, educational work like this study is a promising first step.
References
Garg A. Lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab. 2011;96(11):3313–25. https://doi.org/10.1210/jc.2011-1159.
Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350(12):1220–34. https://doi.org/10.1056/NEJMra025261.
National Organization for Rare Disorders (NORD). The physician’s guide to lipodystrophy disorders. 2014. https://rarediseases.org/physician-guide/lipodystrophy-disorders/. Accessed 9 May 2018.
Rodriguez AJ, Mastronardi CA, Paz-Filho GJ. New advances in the treatment of generalized lipodystrophy: role of metreleptin. Ther Clin Risk Manage. 2015;11:1391–400. https://doi.org/10.2147/tcrm.s66521.
Javor ED, Cochran EK, Musso C, Young JR, Depaoli AM, Gorden P. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54(7):1994–2002.
Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–8. https://doi.org/10.1056/NEJMoa012437.
Chiquette E, Oral EA, Garg A, Araújo-Vilar D, Dhankhar P. Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges. Diabetes Metab Syndr Obes. 2017;10:375–83. https://doi.org/10.2147/dmso.S130810.
Budych K, Helms TM, Schultz C. How do patients with rare diseases experience the medical encounter? Exploring role behavior and its impact on patient-physician interaction. Health Policy. 2012;105(2–3):154–64. https://doi.org/10.1016/j.healthpol.2012.02.018.
EURODIS-Rare Disease Europe. Juggling care and daily life: the balancing act of the rare disease community. A rare barometer survey. Paris: EURODIS-Rare Disease Europe; 2017.
Eatough V, Santini H, Eiser C, Goller M-L, Krysa W, Paduanello M, et al. The personal experience of parenting a child with juvenile Huntington’s disease: perceptions across Europe. Eur J Hum Genet. 2013;21(10):1042.
Strehle E, Middlemiss P. Children with 4q-syndrome: the parents’ perspective. Genet Couns. 2007;18(2):189–200.
Rivard MT, Mastel-Smith B. The lived experience of fathers whose children are diagnosed with a genetic disorder. J Obstet Gynecol Neonatal Nurs. 2014;43(1):38–49.
Schieppati A, Henter JI, Daina E, Aperia A. Why rare diseases are an important medical and social issue. Lancet. 2008;371(9629):2039–41. https://doi.org/10.1016/s0140-6736(08)60872-7.
Jalal Eldin A, Akinci B, Meral R, Rus D, Swaidan M, Hench R, et al. MON-101 The LD Lync Study: natural history study of lipodystrophy syndromes: early lessons from the pilot data. J Endocrine Soc. 2019. https://doi.org/10.1210/js.2019-MON-101.
Cook K, Adamski K, Gomes A, Tuttle E, Kalden H, Cochran E, et al. Effects of metreleptin on patient outcomes and quality of life in generalized and partial lipodystrophy. J Endocrine Soc. 2021. https://doi.org/10.1210/jendso/bvab019.
Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Elo S, Kääriäinen M, Kanste O, Pölkki T, Utriainen K, Kyngäs H. Qualitative content analysis: a focus on trustworthiness. SAGE Open. 2014;4(1):2158244014522633.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57. https://doi.org/10.1093/intqhc/mzm042.
von der Lippe C, Diesen PS, Feragen KB. Living with a rare disorder: a systematic review of the qualitative literature. Mol Gen Genom Med. 2017;5(6):758–73. https://doi.org/10.1002/mgg3.315.
Cook K, Stears A, Araujo-Vilar D, Santini F, O’Rahilly S, Ceccarini G, et al. Real-world experience of patients with generalized and partial lipodystrophy enrolled in the metreleptin early access program. In: 21st European Congress of Endocrinology; 18–21 May 2019; Lyon.
Akinci B, Oral EA, Neidert A, Rus D, Cheng WY, Thompson-Leduc P, et al. Comorbidities and survival in patients with lipodystrophy: an international chart review study. J Clin Endocrinol Metab. 2019;104(11):5120–35. https://doi.org/10.1210/jc.2018-02730.
Kent EE, Ambs A, Mitchell SA, Clauser SB, Smith AW, Hays RD. Health-related quality of life in older adult survivors of selected cancers: data from the SEER-MHOS linkage. Cancer. 2015;121(5):758–65.
Ge T, Fan J, Yang W, Cui R, Li B. Leptin in depression: a potential therapeutic target. Cell Death Dis. 2018;9(11):1096. https://doi.org/10.1038/s41419-018-1129-1.
Doyle M. Peer support and mentorship in a US rare disease community: findings from the Cystinosis in Emerging Adulthood Study. Patient. 2015;8(1):65–73. https://doi.org/10.1007/s40271-014-0085-9.
Acknowledgements
We thank Christian Frois for contributing to the study conception, design of the data collection instrument, and collection of data. We are also grateful to the participants of this study for their time and valuable contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Aegerion Pharmaceuticals Inc. (a wholly owned subsidiary of Amryt Pharmaceuticals) and Amryt Pharmaceuticals.
Conflicts of interest/Competing interests
Aparna Gomes, Keziah Cook, Alex Wong, and Edward Tuttle are employees of Analysis Group, Inc., which has received consultancy fees. Rebecca Sanders is the Co-Founder and Chair of Lipodystrophy UK. Andra Stratton is the Co-Founder and President of Lipodystrophy United.
Ethics approval
The New England Independent Review Board reviewed the study protocol and discussion guide prior to granting an exemption from formal institutional review board review and approval as this study only involves the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, and interview procedures and information was not recorded in such a manner that human subjects can be identified. UK National Health Service Research Ethics Committee approval was not required for participants located in England.
Consent to participate
Verbal informed consent was obtained from all individual participants included in the study prior to the interviews.
Consent for publication
Verbal informed consent was obtained from all individual participants to publish non-identifying information collected during the interview.
Availability of data and material
Data sharing is not applicable as no datasets were generated or analyzed during the current study. Interview transcripts are not publicly available and will not be shared by the corresponding author to protect participant identities.
Code availability
The codebook generated during this study is available from the corresponding author on reasonable request.
Author Contributions
AG, KC, and ET contributed to the study conception and design. ET contributed to material preparation and data collection. RS contributed to reviewing the interview questions during the design phase and recruiting participants from the patient community. Data analysis were performed by AG, KC, and AW. The first draft of the manuscript was written by AG, KC, AW, and ET and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gomes, A., Cook, K., Wong, A. et al. Experiences and Perspectives of Patients with Non-HIV-Associated Lipodystrophies and Their Caregivers: A Qualitative Study. Patient 14, 673–685 (2021). https://doi.org/10.1007/s40271-021-00511-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-021-00511-5