Abstract
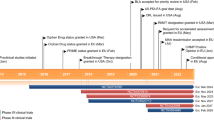
Etranacogene dezaparvovec (etranacogene dezaparvovec-drlb; Hemgenix®) is an adeno-associated virus vector-based gene therapy being developed by uniQure and CSL Behring for the treatment of haemophilia B. In November 2022, etranacogene dezaparvovec was approved in the USA for the treatment of haemophilia B [congenital factor IX (FIX) deficiency] in adults who are currently using FIX prophylaxis therapy, have current or historical life-threatening haemorrhage or have repeated, serious spontaneous bleeding episodes. In December 2022, etranacogene dezaparvovec also received positive opinion in the EU for the treatment of haemophilia B. This article summarizes the milestones in the development of etranacogene dezaparvovec leading to this first approval.
Similar content being viewed by others
References
Bolous NS, Bhatt N, Bhakta N, et al. Gene therapy and hemophilia: where do we go from here? J Blood Med. 2022;13:559–80.
Shah J, Kim H, Sivamurthy K, et al. Comprehensive analysis and prediction of long-term durability of factor IX activity following etranacogene dezaparvovec gene therapy in the treatment of hemophilia B. Curr Med Res Opin. 2022;39(2):227–37.
Thornburg CD. Etranacogene dezaparvovec for hemophilia B gene therapy. Ther Adv Rare Dis. 2021;2:1–14.
US Food & Drug Administration. FDA approves first gene therapy to treat adults with hemophilia B [media release]. 22 Nov 2022. https://www.fda.gov/.
European Medicines Agency. First gene therapy to treat haemophilia B [media release]. 16 Dec 2022. https://www.ema.europa.eu/en/news/first-gene-therapy-treat-haemophilia-b.
uniQure. uniQure announces FDA acceptance of Biologics License Application for etranacogene dezaparvovec under priority review [media release]. 25 May 2022. http://www.uniqure.com.
CSL Behring. Hemgenix® (etranacogene dezaparvovec-drlb) suspension, for intravenous infusion: US prescribing information; 2022. https://labeling.cslbehring.com/PI/US/Hemgenix/EN/Hemgenix-Prescribing-Information.pdf. Accessed 24 Nov 2022.
uniQure. uniQure announces license agreement with CSL Behring to commercialize hemophilia B gene therapy [media release]. 25 Jun 2020. http://www.uniqure.com.
CSL Behring, uniQure. CSL Behring announces global commercialization and license agreement with uniQure [media release]. 7 May 2021. http://www.csl.com.
United States securities and exchange commission. uniQure 2021, Form 10-K. Internet-Doc; 2022. https://www.sec.gov/ix?doc=/Archives/edgar/data/1590560/000155837022002050/qure-20211231x10k.htm. Accessed 20 Dec 2022.
uniQure. uniQure strengthens intellectual property portfolio with acquisition of patent family providing broad protection of the hyperactive Padua variant of factor IX (FIX-Padua) [media release]. 26 Oct 2017. http://www.uniqure.com.
uniQure. uniQure announces the issuance of new patents providing broad protection of the Padua variant of factor IX in gene therapy [media release]. 31 May 2018. http://www.uniqure.com.
Spronck EA, Liu YP, Lubelski J, et al. Enhanced factor IX activity following administration of AAV5-R338L “Padua” factor IX versus AAV5 WT human factor IX in NHPs. Mol Ther Methods Clin Dev. 2019;15:221–31.
Miesbach W, Leebeek FWG, Recht M, et al. Final analysis from the pivotal phase 3 HOPE-B gene therapy trial: stable steady-state efficacy and safety of etranacogene dezaparvovec in adults with severe or moderately severe hemophilia b [abstract no. PO143 and presentation]. Haemophilia. 2022;28(Suppl 1):99–100.
Pipe SW, Leebeek FWG, Recht M, et al. Stable hemostatic correction and improved hemophilia-related quality of life: final analysis from the pivotal phase 3 HOPE-B trial of etranacogene dezaparvovec [abstract no. 1192]. Mol Ther. 2022;30(4 Suppl):552–3.
Recht M, Leebeek FWG, Miesbach W, et al. Clinical outcomes in patients with and without pre-existing neutralizing antibodies to the vector: 6 month data from the phase 3 HOPE-B gene therapy trial of etranacogene dezaparvovec [abstract no. 88]. Mol Ther. 2021;29(4 Suppl 1):45.
Pipe S, Leebeek F, Recht M, et al. 52 week efficacy and safety of etranacogene dezaparvovec in adults with severe or moderate-severe hemophilia B: data from the phase 3 HOPE-B gene therapy trial [abstract no. PB0653]. Res Pract Thromb Haemost. 2021;5(Suppl 2).
Von Drygalski A, Giermasz A, Castaman G, et al. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 2019;3(21):3241–7.
Gomez E, Castaman G, Key NS, et al. Multiple-year durability data from a phase 2b trial of gene therapy with etranacogene dezaparvovec in patients with hemophilia b [abstract no. PO098]. Haemophilia. 2022;28(Suppl):74–5.
von Drygalski A, Giermasz A, Castaman G, et al. Etranacogene dezaparvovec (AAV5-PADUA HFIX variant) in adults with severe or moderate-severe hemophilia b: two year data from a phase 2b trial [abstract no. ABS100]. Haemophilia. 2021;27(Suppl 2):72–3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Young-A Heo is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heo, YA. Etranacogene Dezaparvovec: First Approval. Drugs 83, 347–352 (2023). https://doi.org/10.1007/s40265-023-01845-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01845-0