Abstract
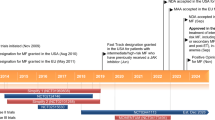
Pacritinib (VONJO™) is an orally administered, small molecule kinase inhibitor being developed by CTI BioPharma for the treatment of myelofibrosis and graft-versus-host disease. Pacritinib received its first approval in February 2022 in the USA for the treatment of adults with intermediate- or high-risk primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis with a platelet count below 50 × 109/L. The accelerated approval was based on results from the randomized, active-controlled, phase III PERSIST-2 trial, in which spleen volume reduction was demonstrated in pacritinib recipients. This article summarizes the milestones in the development of pacritinib leading to this first approval for myelofibrosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Patel AA, Odenike O. The next generation of JAK inhibitors: an update on fedratinib, momelotonib, and pacritinib. Curr Hematol Malig Rep. 2020;15(6):409–18.
Verstovsek S, Komrokji RS. A comprehensive review of pacritinib in myelofibrosis. Future Oncol. 2015;11(20):2819–30.
Singer JW, Al-Fayoumi S, Ma H, et al. Comprehensive kinase profile of pacritinib, a nonmyelosuppressive Janus kinase 2 inhibitor. J Exp Pharmacol. 2016;8:11–9.
CTI BioPharma. CTI BioPharma announces FDA accelerated approval of VONJO™ (pacritinib) for the treatment of adult patients with myelofibrosis and thrombocytopenia [media release]. 1 Mar 2022.
US Food & Drug Administration. FDA approves drug for adults with rare form of bone marrow disorder [media release]. 28 Feb 2022.
CTI BioPharma. VONJO™ (pacritinib) capsules, for oral use: US prescribing information. 2022. https://www.accessdata.fda.gov/. Accessed 27 Mar 2022.
S*BIO Pte Ltd, Cell Therapeutics Inc. Cell Therapeutics enters into agreement to acquire pacritinib, a novel highly selective JAK2 inhibitor phase 3 candidate for myelofibrosis [media release]. 20 Apr 2012.
Cell Therapeutics Inc. Cell Therapeutics completes acquisition of pacritinib a highly selective JAK2 inhibitor [media release]. 5 Jun 2012.
Onyx Pharmaceuticals Inc. Onyx Pharmaceuticals acquires option to license novel highly specific JAK2 inhibitors from S*BIO [media release]. 7 Jan 2009.
S*BIO Pte Ltd, Onyx Pharmaceuticals Inc. S*BIO and Onyx Pharmaceuticals announce expanded development collaboration for JAK2 inhibitors [media release]. 5 May 2010.
S*BIO Pte Ltd. S*BIO restructures agreement with Onyx for JAK2 inhibitor program [media release]. 5 May 2011.
S*BIO Pte Ltd. S*BIO enters research collaboration with Tan Tock Seng Hospital for therapeutic and biomarker evaluation of JAK2 inhibitor SB1518 [media release]. 19 Dec 2008.
CTI BioPharma. United States Securities and Exchange Commission: Form 10-K. 2020. https://www.sec.gov/. Accessed 24 Mar 2022
Hart S, Goh KC, Novotny-Diermayr V, et al. SB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the treatment of myeloid and lymphoid malignancies. Leukemia. 2011;25(11):1751–9.
Al-Fayoumi S, Hashiguchi T, Shirakata Y, et al. Pilot study of the antifibrotic effects of the multikinase inhibitor pacritinib in a mouse model of liver fibrosis. J Exp Pharmacol. 2018;10:9–17.
Verstovsek S, Odenike O, Singer JW, et al. Phase 1/2 study of pacritinib, a next generation JAK2/FLT3 inhibitor, in myelofibrosis or other myeloid malignancies. J Hematol Oncol. 2016;9(1):137.
Jayaraman R, Pasha MK, Williams A, et al. Metabolism and disposition of pacritinib (SB1518), an orally active Janus kinase 2 inhibitor in preclinical species and humans. Drug Metab Lett. 2015;9(1):28–47.
Mascarenhas J, Hoffman R, Talpaz M, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4(5):652–9.
US Food & Drug Administration. Center for Drug Evaluation and Research application number: 208712Orig1s000 (integrated review of pacritinib). 2021. https://www.accessdata.fda.gov/. Accessed 12 Apr 2022.
Mesa RA, Vannucchi AM, Mead A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4(5):e225–36.
Verstovsek S, Mesa R, Talpaz M, et al. Retrospective analysis of pacritinib in patients with myelofibrosis and severe thrombocytopenia. Haematologica. 2021. https://doi.org/10.3324/haematol.2021.279415.
Tremblay D, Mesa R, Scott B, et al. Pacritinib demonstrates spleen volume reduction in patients with myelofibrosis independent of JAK2V617F allele burden. Blood Adv. 2020;4(23):5929–35.
Te Boekhorst PA, Van Der Holt B, Meijer E, et al. Interim analysis of a phase II trial (HOVON-134) in myelofibrosis patients (primary, post-ET or post PV-MF) treated with the JAK2 inhibitor pacritinib before RIC allogeneic stem cell transplantation. HemaSphere. 2020;4(Suppl. 1):502.
Gerds AT, Savona MR, Scott BL, et al. Determining the recommended dose of pacritinib: results from the PAC203 dose-finding trial in advanced myelofibrosis. Blood Adv. 2020;4(22):5825–35.
Komrokji RS, Seymour JF, Roberts AW, et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosis. Blood. 2015;125(17):2649–55.
Pidala J, Walton K, Elmariah H, et al. Pacritinib combined with sirolimus and low-dose tacrolimus for GVHD prevention after allogeneic hematopoietic cell transplantation: preclinical and phase I trial results. Clin Cancer Res. 2021;27(10):2712–22.
Data on file, CTI BioPharma. 2022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yvette Lamb is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lamb, Y.N. Pacritinib: First Approval. Drugs 82, 831–838 (2022). https://doi.org/10.1007/s40265-022-01718-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01718-y