Abstract
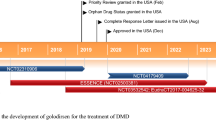
Casimersen (Amondys 45™) is an antisense oligonucleotide of the phosphorodiamidate morpholino oligomer subclass developed by Sarepta Therapeutics for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a mutation in the DMD gene that is amenable to exon 45 skipping. Administered by intravenous infusion, casimersen is designed to bind to exon 45 of the DMD gene pre-mRNA, resulting in skipping of this exon during mRNA processing, intended to allow for production of an internally truncated but functional dystrophin protein in patients with DMD. Casimersen received its first approval on 25 February 2021, in the USA, for the treatment of DMD in patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping. The approval, granted under the US FDA Accelerated Approval Program, was based on an observed increase in dystrophin production in skeletal muscle in patients treated with casimersen. Casimersen is continuing in phase III development for the treatment of DMD in several other countries worldwide. This article summarises the milestones in the development of casimersen leading to this first approval for DMD. As with other approvals under the Accelerated Approval Program, continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.References
Duan D, Goemans N, Takeda S, et al. Duchenne muscular dystrophy. Nat Rev Dis Primers. 2021;7(1):13.
Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17(3):251–67.
Datta N, Ghosh PS. Update on muscular dystrophies with focus on novel treatments and biomarkers. Curr Neurol Neurosci Rep. 2020;20(14):1–12.
Rodrigues M, Yokota T. An overview of recent advances and clinical applications of exon skipping and splice modulation for muscular dystrophy and various genetic diseases. In: Yokota T, Maruyama R, editors. Exon skipping and inclusion therapies. Methods in molecular biology, vol. 1828. New York: Humana Press; 2018.
Verhaart IEC, Aartsma-Rus A. Therapeutic developments for Duchenne muscular dystrophy. Nat Rev Neurol. 2019;15(7):373–86.
US FDA. Amondys 45 (casimersen) injection, for intravenous use: US prescribing information. 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213026lbl.pdf. Accessed 18 Mar 2021.
Sarepta Therapeutics Inc. Sarepta Therapeutics announces FDA approval of Amondys 45™ (casimersen) injection for the treatment of Duchenne muscular dystrophy (DMD) in patients amenable to skipping exon 45 [media release]. 2021. https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-fda-approval-amondys-45tm. Accessed 18 Mar 2021.
US FDA. FDA approves targeted treatment for rare Duchenne muscular dystrophy mutation [media release]. 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-targeted-treatment-rare-duchenne-muscular-dystrophy-mutation-0. Accessed 18 Mar 2021.
Sarepta Therapeutics. Sarepta Therapeutics and University of Western Australia announce exclusive worldwide licensing agreement for exon-skipping program in Duchenne muscular dystrophy [media release]. 2013. https://investorrelations.sarepta.com/static-files/e20a099e-f99d-4237-8651-fa9c6d479090. Accessed 18 Mar 2021.
United States Securities and Exhange Commission. Sarepta Therapeutics, Inc.—Form 10-K. 2018. https://www.sec.gov/Archives/edgar/data/873303/000156459019005170/srpt-10k_20181231.htm. Accessed 18 Mar 2021.
Wagner K, Kuntz N, Koenig E, et al. Casimersen treatment in patients with Duchenne muscular dystrophy: safety, tolerability, and pharmacokinetics over 144 weeks of treatment [poster P.288]. In: World Muscle Society Virtual Congress. 2020.
Kuntz N, Wagner K, East L, et al. Casimersen treatment in eligible patients with Duchenne muscular dystrophy: safety, tolerability, and pharmacokinetics over 144 weeks of treatment [abstract no. P.288]. Neuromuscul Disord. 2020;30(Suppl 1):S130–1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. M. Shirley is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Shirley, M. Casimersen: First Approval. Drugs 81, 875–879 (2021). https://doi.org/10.1007/s40265-021-01512-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01512-2