Abstract
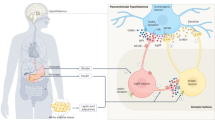
Setmelanotide (IMCIVREE™, Rhythm Pharmaceuticals) is a melanocortin-4 (MC4) receptor agonist developed for the treatment of obesity arising from proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency. The drug has received its first approval in the USA for chronic weight management in patients 6 years and older with obesity caused by POMC, PCSK1 and LEPR deficiency and has been granted PRIority MEdicines (PRIME) designation by the European Medicines Agency for the treatment of obesity and the control of hunger associated with deficiency disorders of the MC4 receptor pathway. Setmelanotide is also being developed in other rare genetic disorders associated with obesity including Bardet–Biedl Syndrome, Alström Syndrome, POMC and other MC4R pathway heterozygous deficiency obesities, and POMC epigenetic disorders. This article summarizes the milestones in the development of setmelanotide leading to this first approval for obesity caused by POMC, PCSK1 and LEPR deficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Rhythm Pharmaceuticals. Rhythm Pharmaceuticals announces FDA approval of IMCIVREETM (setmelanotide) as first-ever therapy for chronic weight management in patients with obesity due to POMC, PCSK1 or LEPR deficiency [media release]. 27 Nov 2020. http://www.rhythmtx.com/.
Ryan DH. Setmelanotide: what does it mean for clinical care of patients with obesity? Lancet Diabetes Endocrinol. 2020;8(12):933–5.
Kuhnen P, Clement K, Wiegand S, et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016;375(3):240–6.
Rhythm Pharmaceuticals. Setmelanotide (IMCIVREE™): US prescribing information. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213793s000lbl.pdf. Accessed 2 Dec 2020.
US Food and Drug Administration. FDA approves first treatment for weight management for people with certain rare genetic conditions [media release]. 27 Nov 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-first-treatment-weight-management-people-certain-rare-genetic-conditions.
Ipsen Limited. Ipsen grants Rhythm exclusive worldwide license for two programs in the field of metabolic disorders [media release]. 12 Mar 2010. http://www.ipsen.com.
Rhythm Pharmaceuticals. Rhythm and Camurus announce license agreement for extended release FluidCrystal setmelanotide [media release]. 5 Jan 2016. http://www.rhythmtx.com.
Roubert P, Dubern B, Plas P, et al. Novel pharmacological MC4R agonists can efficiently activate mutated MC4R from obese patient with impaired endogenous agonist response. J Endocrinol. 2010;207(2):177–83.
Thompson Z, Shah BP, Low MJ. Chronic treatment of juvenile hypothalamic POMC-deficient mice with RM-493 prevents the development of obesity [abstract no. SAT-299]. J Endocr Soc. 2020;4(Suppl):A166–7.
Bischof JM, Van Der Ploeg LHT, Colmers WF, et al. Magel2-null mice are hyper-responsive to setmelanotide, a melanocortin 4 receptor agonist. Br J Pharmacol. 2016;2016:2614–21.
Stephenson E, McAllan L, Stayton A, et al. Setmelanotide (RM-493) reduces food intake and rapidly induces weight loss in a mouse model of Alström syndrome [abstract no. MON-LB019]. J Endocr Soc. 2019;3(Suppl 1).
Kievit P, Halem H, Marks DL, et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62(2):490–7.
Chen KY, Muniyappa R, Abel BS, et al. RM-493, a melanocortin-4 receptor (MC4R) agonist increases resting energy expenditure in obese individuals. J Clin Endocrinol Metab. 2015;100(4):1639–45.
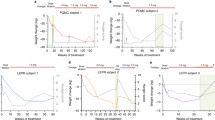
Clement K, van den Akker E, Argente J, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8(12):960–70.
Clement K, Biebermann H, Farooqi IS, et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med. 2018;24(5):551–5.
Rhythm Pharmaceuticals. Rhythm Pharmaceuticals announces positive topline results from pivotal phase 3 clinical trial evaluating setmelanotide in Bardet-Biedl and Alström syndromes [media release]. 22 Dec 2020. https://ir.rhythmtx.com/.
Haws R, Brady S, Davis E, et al. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes Metab. 2020;22(11):2133–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Markham is a contracted employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Markham, A. Setmelanotide: First Approval. Drugs 81, 397–403 (2021). https://doi.org/10.1007/s40265-021-01470-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01470-9