Abstract
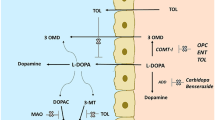
Inhibitors of catechol-O-methyltransferase (COMT) are commonly used as an adjunct to levodopa in patients with Parkinson’s disease (PD) for the amelioration of wearing-off symptoms. This narrative review aims to discuss the role of COMT inhibitors on peripheral levodopa metabolism and continuous brain delivery of levodopa, and to describe their metabolic properties. Oral application of levodopa formulations with a dopa decarboxylase inhibitor (DDI) results in fluctuating levodopa plasma concentrations, predominantly due to the short half-life of levodopa and its slowing of gastric emptying. Following transport across the blood–brain barrier and its metabolic conversion to dopamine, these peripheral ‘ups and downs’ of levodopa are reflected in fluctuating dopamine levels in the synaptic cleft between presynaptic and postsynaptic dopaminergic neurons of the nigrostriatal system. As a result, pulsatile postsynaptic dopaminergic stimulation takes place and results in the occurrence of motor complications, such as wearing-off and dyskinesia. More continuous plasma behaviour was observed after the combination of levodopa/DDI formulations with COMT inhibitors. These compounds also weaken a levodopa/DDI-related homocysteine increase, as biomarker for an impaired methylation capacity, which is involved in an elevated oxidative stress exposure. These findings favour the concept of chronic levodopa/DDI application with concomitant inhibition of COMT and monoamine oxidase, since deamination of dopamine via this enzyme also generates free radicals. This triple combination is suggested as standard levodopa application in patients with PD who need levodopa, if they will tolerate it.


Similar content being viewed by others
References
Rajput AH, Birdi S. Epidemiology of Parkinson’s disease. Parkinsonism Relat Disord. 1997;3(4):175–86.
Brooks DJ. Examining Braak’s hypothesis by imaging Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S83–8.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–4.
Milber JM, Noorigian JV, Morley JF, Petrovitch H, White L, Ross GW, et al. Lewy pathology is not the first sign of degeneration in vulnerable neurons in Parkinson disease. Neurology. 2012;79(24):2307–14.
Stoddard SL. The adrenal medulla and Parkinson’s disease. Rev Neurosci. 1994;5(4):293–307.
Przuntek H, Müller T, Riederer P. Diagnostic staging of Parkinson’s disease: conceptual aspects. J Neural Transm. 2004;111(2):201–16.
Lim SY, Fox SH, Lang AE. Overview of the extranigral aspects of Parkinson disease. Arch Neurol. 2009;66(2):167–72.
Lim SY, Lang AE. The nonmotor symptoms of Parkinson’s disease—an overview. Mov Disord. 2010;25(Suppl 1):S123–30.
Siderowf A, Lang AE. Premotor Parkinson’s disease: concepts and definitions. Mov Disord. 2012;27(5):608–16.
Dickson DW. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med. 2012;2(8):a009258.
Weiner WJ. There is no Parkinson disease. Arch Neurol. 2008;65(6):705–8.
Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol. 2013;8(1):189–201.
Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18(9):685–716.
Naoi M, Maruyama W, Yi H, Inaba K, Akao Y, Shamoto-Nagai M. Mitochondria in neurodegenerative disorders: regulation of the redox state and death signaling leading to neuronal death and survival. J Neural Transm. 2009;116(11):1371–81.
Riederer P, Gerlach M, Müller T, Reichmann H. Relating mode of action to clinical practice: dopaminergic agents in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(8):466–79.
Müller T. Detoxification and antioxidative therapy for levodopa-induced neurodegeneration in Parkinson’s disease. Expert Rev Neurother. 2013;13(6):707–18.
Müller T. Drug therapy in patients with Parkinson’s disease. Transl Neurodegener. 2012;1(1):1–10.
Müller T. Pharmacokinetic/pharmacodynamic evaluation of rasagiline mesylate for Parkinson’s disease. Expert Opin Drug Metab Toxicol. 2014;10(10):1423–32.
Birkmayer W, Hornykiewicz O. The effect of l-3,4-dihydroxyphenylalanine (=DOPA) on akinesia in parkinsonism. 1961. Wien Klin Wochenschr. 2001;113(22):851–4.
Müller T, Russ H. Levodopa, motor fluctuations and dyskinesia in Parkinson’s disease. Expert Opin Pharmacother. 2006;7(13):1715–30.
Rodnitzky RL, Narayanan NS. Amantadine’s role in the treatment of levodopa-induced dyskinesia. Neurology. 2014;82(4):288–9.
Stocchi F, Tagliati M, Olanow CW. Treatment of levodopa-induced motor complications. Mov Disord. 2008;23(Suppl 3):S599–612.
Nutt JG, Chung KA, Holford NH. Dyskinesia and the antiparkinsonian response always temporally coincide: a retrospective study. Neurology. 2010;74(15):1191–7.
Pearce RK, Heikkila M, Linden IB, Jenner P. l-dopa induces dyskinesia in normal monkeys: behavioural and pharmacokinetic observations. Psychopharmacology (Berl). 2001;156(4):402–9.
Cenci MA, Konradi C. Maladaptive striatal plasticity in l-DOPA-induced dyskinesia. Prog Brain Res. 2010;183:209–33.
Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of amantadine benefit on dyskinesia of severe Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75(1):141–3.
Politis M, Wu K, Molloy S, Bain G, Chaudhuri KR, Piccini P. Parkinson’s disease symptoms: the patient’s perspective. Mov Disord. 2010;25(11):1646–51.
Olanow CW, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, et al. Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord. 2013;28(8):1064–71.
Smith LA, Jackson MJ, Hansard MJ, Maratos E, Jenner P. Effect of pulsatile administration of levodopa on dyskinesia induction in drug-naive MPTP-treated common marmosets: effect of dose, frequency of administration, and brain exposure. Mov Disord. 2003;18(5):487–95.
Foley P, Mizuno Y, Nagatsu T, Sano A, Youdin MBH, McGeer P, et al. The l-DOPA story—an early Japanese contribution. Parkinsonism Relat Disord. 2000;6(1):1.
Pivac N, Pregelj P, Nikolac M, Zupanc T, Nedic G, Muck SD, et al. The association between catechol-O-methyl-transferase Val108/158Met polymorphism and suicide. Genes Brain Behav. 2011;10(5):565–9.
Schosser A, Calati R, Serretti A, Massat I, Kocabas NA, Papageorgiou K, et al. The impact of COMT gene polymorphisms on suicidality in treatment resistant major depressive disorder—a European multicenter study. Eur Neuropsychopharmacol. 2012;22(4):259–66.
Wardle MC, Hart AB, Palmer AA, de Wit H. Does COMT genotype influence the effects of d-amphetamine on executive functioning? Genes Brain Behav. 2013;12(1):13–20.
Kaakkola S. Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson’s disease. Drugs. 2000;59(6):1233–50.
Mannisto PT, Tuomainen P, Tuominen RK. Different in vivo properties of three new inhibitors of catechol O-methyltransferase in the rat. Br J Pharmacol. 1992;105(3):569–74.
Müller T, Kolf K, Ander L, Woitalla D, Muhlack S. Catechol-O-methyltransferase inhibition improves levodopa-associated strength increase in patients with Parkinson disease. Clin Neuropharmacol. 2008;31(3):134–40.
Tornwall M, Kaakkola S, Tuomainen P, Kask A, Mannisto PT. Comparison of two new inhibitors of catechol O-methylation on striatal dopamine metabolism: a microdialysis study in rats. Br J Pharmacol. 1994;112(1):13–8.
Zurcher G, Colzi A, Da PM. Ro 40-7592: inhibition of COMT in rat brain and extracerebral tissues. J Neural Transm Suppl. 1990;32:375–80.
Nutt JG, Carter JH, Lea ES, Woodward WR. Motor fluctuations during continuous levodopa infusions in patients with Parkinson’s disease. Mov Disord. 1997;12(3):285–92.
Kuoppamaki M, Korpela K, Marttila R, Kaasinen V, Hartikainen P, Lyytinen J, et al. Comparison of pharmacokinetic profile of levodopa throughout the day between levodopa/carbidopa/entacapone and levodopa/carbidopa when administered four or five times daily. Eur J Clin Pharmacol. 2009;65(5):443–55.
Müller T, Erdmann C, Muhlack S, Bremen D, Przuntek H, Woitalla D. Inhibition of catechol-O-methyltransferase contributes to more stable levodopa plasma levels. Mov Disord. 2006;21(3):332–6.
Müller T, Erdmann C, Bremen D, Schmidt WE, Muhlack S, Woitalla D, et al. Impact of gastric emptying on levodopa pharmacokinetics in Parkinson disease patients. Clin Neuropharmacol. 2006;29(2):61–7.
Müller T, Erdmann C, Muhlack S, Bremen D, Przuntek H, Goetze O, et al. Pharmacokinetic behaviour of levodopa and 3-O-methyldopa after repeat administration of levodopa/carbidopa with and without entacapone in patients with Parkinson’s disease. J Neural Transm. 2006;113(10):1441–8.
Müller T. The impact of COMT-inhibition on gastrointestinal levodopa absorption in patients with Parkinson’s disease. Clin Med Insights Ther. 2010;2:155–68.
Müller T. Levodopa/carbidopa and entacapone in the treatment of Parkinson’s disease: efficacy, safety and patient preference. Patient Prefer Adherence. 2009;3:51–9.
Müller T. Motor complications, levodopa metabolism and progression of Parkinson’s disease. Expert Opin Drug Metab Toxicol. 2011;7(7):847–55.
Nyholm D, Nilsson Remahl AI, Dizdar N, Constantinescu R, Holmberg B, Jansson R, et al. Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology. 2005;64(2):216–23.
Ekesbo A, Rydin E, Torstenson R, Sydow O, Laengstrom B, Tedroff J. Dopamine autoreceptor function is lost in advanced Parkinson’s disease. Neurology. 1999;52(1):120–5.
Cenci MA. Dopamine dysregulation of movement control in l-DOPA-induced dyskinesia. Trends Neurosci. 2007;30(5):236–43.
Calabresi P, Di FM, Ghiglieri V, Picconi B. Molecular mechanisms underlying levodopa-induced dyskinesia. Mov Disord. 2008;23(Suppl 3):S570–9.
Jugel C, Ehlen F, Taskin B, Marzinzik F, Müller T, Klostermann F. Neuropathy in Parkinson’s disease patients with intestinal levodopa infusion versus oral drugs. PLoS One. 2013;8(6):e66639.
Klostermann F, Jugel C, Müller T, Marzinzik F. Malnutritional neuropathy under intestinal levodopa infusion. J Neural Transm. 2012;119(3):369–72.
Meiler B, Andrich J, Müller T. Rapid switch from oral antiparkinsonian combination drug therapy to duodenal levodopa infusion. Mov Disord. 2008;23(1):145–6.
Klostermann F, Jugel C, Bomelburg M, Marzinzik F, Ebersbach G, Müller T. Severe gastrointestinal complications in patients with levodopa/carbidopa intestinal gel infusion. Mov Disord. 2012;27(13):1704–5.
Kleedorfer B, Lees AJ, Stern GM. Subcutaneous and sublingual levodopa methyl ester in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1991;54(4):373.
Lee YH, Kim KH, Yoon IK, Lee KE, Chun IK, Rhie JY, et al. Pharmacokinetic evaluation of formulated levodopa methyl ester nasal delivery systems. Eur J Drug Metab Pharmacokinet. 2014;39(4):237–42.
Dupont E, Burgunder JM, Findley LJ, Olsson JE, Dorflinger E. Tolcapone added to levodopa in stable parkinsonian patients: a double-blind placebo-controlled study. Tolcapone in Parkinson’s Disease Study Group II (TIPS II). Mov Disord. 1997;12(6):928–34.
Block G, Liss C, Reines S, Irr J, Nibbelink D. Comparison of immediate-release and controlled release carbidopa/levodopa in Parkinson’s disease. A multicenter 5-year study. The CR First Study Group. Eur Neurol. 1997;37(1):23–7.
Piccini P, Brooks DJ, Korpela K, Pavese N, Karlsson M, Gordin A. The catechol-O-methyltransferase (COMT) inhibitor entacapone enhances the pharmacokinetic and clinical response to Sinemet CR in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2000;68(5):589–94.
Smith LA, Jackson MJ, Al-Barghouthy G, Rose S, Kuoppamaki M, Olanow W, et al. Multiple small doses of levodopa plus entacapone produce continuous dopaminergic stimulation and reduce dyskinesia induction in MPTP-treated drug-naive primates. Mov Disord. 2005;20(3):306–14.
Stocchi F, Rascol O, Kieburtz K, Poewe W, Jankovic J, Tolosa E, et al. Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann Neurol. 2010;68(1):18–27.
Müller T. Pharmacokinetic considerations for the use of levodopa in the treatment of Parkinson disease: focus on levodopa/carbidopa/entacapone for treatment of levodopa-associated motor complications. Clin Neuropharmacol. 2013;36(3):84–91.
Muhlack S, Herrmann L, Salmen S, Müller T. Fewer fluctuations, higher maximum concentration and better motor response of levodopa with catechol-O-methyltransferase inhibition. J Neural Transm. 2014;121(11):1357–66.
Müller T, Woitalla D, Schulz D, Peters S, Kuhn W, Przuntek H. Tolcapone increases maximum concentration of levodopa. J Neural Transm. 2000;107(1):113–9.
Jorga KM. Pharmacokinetics, pharmacodynamics, and tolerability of tolcapone: a review of early studies in volunteers. Neurology. 1998;50(5 Suppl 5):S31–8.
Hauser RA, Panisset M, Abbruzzese G, Mancione L, Dronamraju N, Kakarieka A. Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/carbidopa in early Parkinson’s disease. Mov Disord. 2009;24(4):541–50.
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351(24):2498–508.
Nyholm D, Askmark H, Aquilonius SM. Stalevo reduction in dyskinesia evaluation in Parkinson’s disease results were expected from a pharmacokinetic viewpoint. Ann Neurol. 2011;69(2):424.
Olanow CW, Kieburtz K, Stocchi F. Initiating levodopa therapy for Parkinson’s disease. Mov Disord. 2014;29(3):430.
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–53.
LeWitt PA, Jennings D, Lyons KE, Pahwa R, Rabinowicz AL, Wang J, et al. Pharmacokinetic–pharmacodynamic crossover comparison of two levodopa extension strategies. Mov Disord. 2009;24(9):1319–24.
Müller T, Woitalla D, Goetze O, Erdmann C. Entacapone improves absorption of a coadministered salt in patients with Parkinson’s disease. Mov Disord. 2008;23(10):1458–61.
Ceravolo R, Piccini P, Bailey DL, Jorga KM, Bryson H, Brooks DJ. 18F-dopa PET evidence that tolcapone acts as a central COMT inhibitor in Parkinson’s disease. Synapse. 2002;43(3):201–7.
Russ H, Müller T, Woitalla D, Rahbar A, Hahn J, Kuhn W. Detection of tolcapone in the cerebrospinal fluid of parkinsonian subjects. Naunyn Schmiedebergs Arch Pharmacol. 1999;360(6):719–20.
De Bonis ML, Tessitore A, Pellecchia MT, Longo K, Salvatore A, Russo A, et al. Impaired transmethylation potential in Parkinson’s disease patients treated with l-Dopa. Neurosci Lett. 2010;468(3):287–91.
Cacciapuoti F. Hyper-homocysteinemia: a novel risk factor or a powerful marker for cardiovascular diseases? Pathogenetic and therapeutical uncertainties. J Thromb Thrombolysis. 2011;32(1):82–8.
Zhang L, Jin Y, Chen M, Huang M, Harvey RG, Blair IA, et al. Detoxication of structurally diverse polycyclic aromatic hydrocarbon (PAH) o-quinones by human recombinant catechol-O-methyltransferase (COMT) via O-methylation of PAH catechols. J Biol Chem. 2011;286(29):25644–54.
Chuang YC, Chuang HY, Lin TK, Chang CC, Lu CH, Chang WN, et al. Effects of long-term antiepileptic drug monotherapy on vascular risk factors and atherosclerosis. Epilepsia. 2012;53(1):120–8.
Müller T. Role of homocysteine in the treatment of Parkinson’s disease. Expert Rev Neurother. 2008;8(6):957–67.
Schwartz RS, Halliday GM, Cordato DJ, Kril JJ. Small-vessel disease in patients with Parkinson’s disease: a clinicopathological study. Mov Disord. 2012;27(12):1506–12.
Müller T, van Laar T, Cornblath DR, Odin P, Klostermann F, Grandas FJ, et al. Peripheral neuropathy in Parkinson’s disease: levodopa exposure and implications for duodenal delivery. Parkinsonism Relat Disord. 2013;19(5):501–7.
Tanner CM, Ross GW, Jewell SA, Hauser RA, Jankovic J, Factor SA, et al. Occupation and risk of parkinsonism: a multicenter case–control study. Arch Neurol. 2009;66(9):1106–13.
Zhang YD, Ke XY, Shen W, Liu Y. Relationship of homocysteine and gene polymorphisms of its related metabolic enzymes with Alzheimer’s disease. Chin Med Sci J. 2005;20(4):247–51.
Zhu BT. Catechol-O-Methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Curr Drug Metab. 2002;3(3):321–49.
Müller T, Kuhn W. Cysteine elevation in levodopa-treated patients with Parkinson’s disease. Mov Disord. 2009;24(6):929–32.
Ho PI, Ashline D, Dhitavat S, Ortiz D, Collins SC, Shea TB, et al. Folate deprivation induces neurodegeneration: roles of oxidative stress and increased homocysteine. Neurobiol Dis. 2003;14(1):32–42.
Zeevalk GD, Razmpour R, Bernard LP. Glutathione and Parkinson’s disease: is this the elephant in the room? Biomed Pharmacother. 2008;62(4):236–49.
Müller T, Muhlack S. Cysteinyl-glycine reduction as marker for Levodopa induced oxidative stress in Parkinson’s disease patients. Mov Disord. 2011;26(3):543–6.
Müller T, Muhlack S. Levodopa-related cysteinyl-glycine and cysteine reduction with and without catechol-O-methyltransferase inhibition in Parkinson’s disease patients. J Neural Transm. 2014;121(6):643–8.
Müller T, Werne B, Fowler B, Kuhn W. Nigral endothelial dysfunction, homocysteine, and Parkinson’s disease. Lancet. 1999;354(9173):126–7.
Müller T, Jugel C, Ehret R, Ebersbach G, Bengel G, Muhlack S, et al. Elevation of total homocysteine levels in patients with Parkinson’s disease treated with duodenal levodopa/carbidopa gel. J Neural Transm. 2011;118(9):1329–33.
Lee ES, Chen H, Soliman KF, Charlton CG. Effects of homocysteine on the dopaminergic system and behavior in rodents. Neurotoxicology. 2005;26(3):361–71.
Nakaso K, Yasui K, Kowa H, Kusumi M, Ueda K, Yoshimoto Y, et al. Hypertrophy of IMC of carotid artery in Parkinson’s disease is associated with l-DOPA, homocysteine, and MTHFR genotype. J Neurol Sci. 2003;207(1–2):19–23.
O’Suilleabhain PE, Sung V, Hernandez C, Lacritz L, Dewey RB Jr, Bottiglieri T, et al. Elevated plasma homocysteine level in patients with Parkinson disease: motor, affective, and cognitive associations. Arch Neurol. 2004;61(6):865–8.
Postuma RB, Lang AE. Homocysteine and levodopa: should Parkinson disease patients receive preventative therapy? Neurology. 2004;63(5):886–91.
Rogers JD, Sanchez-Saffon A, Frol AB, Diaz-Arrastia R. Elevated plasma homocysteine levels in patients treated with levodopa: association with vascular disease. Arch Neurol. 2003;60(1):59–64.
Toth C, Brown MS, Furtado S, Suchowersky O, Zochodne D. Neuropathy as a potential complication of levodopa use in Parkinson’s disease. Mov Disord. 2008;23(13):1850–9.
Ben Shlomo Y, Marmot MG. Survival and cause of death in a cohort of patients with parkinsonism: possible clues to aetiology? J Neurol Neurosurg Psychiatry. 1995;58(3):293–9.
Müller T, Muhlack S. Peripheral COMT inhibition prevents levodopa associated homocysteine increase. J Neural Transm. 2009;116(10):1253–6.
Müller T, Kuhn W. Tolcapone decreases plasma levels of S-adenosyl-l-homocysteine and homocysteine in treated Parkinson’s disease patients. Eur J Clin Pharmacol. 2006;62(6):447–50.
Postuma RB, Espay AJ, Zadikoff C, Suchowersky O, Martin WR, Lafontaine AL, et al. Vitamins and entacapone in levodopa-induced hyperhomocysteinemia: a randomized controlled study. Neurology. 2006;66(12):1941–3.
Zesiewicz TA, Wecker L, Sullivan KL, Merlin LR, Hauser RA. The controversy concerning plasma homocysteine in Parkinson disease patients treated with levodopa alone or with entacapone: effects of vitamin status. Clin Neuropharmacol. 2006;29(3):106–11.
Lamberti P, Zoccolella S, Iliceto G, Armenise E, Fraddosio A, DeMari M, et al. Effects of levodopa and COMT inhibitors on plasma homocysteine in Parkinson’s disease patients. Mov Disord. 2005;20(1):69–72.
Müller T, Woitalla D, Muhlack S. Inhibition of catechol-O-methyltransferase modifies acute homocysteine rise during repeated levodopa application in patients with Parkinson’s disease. Naunyn Schmiedebergs Arch Pharmacol. 2011;383(6):627–33.
Nevrly M, Kanovsky P, Vranova H, Langova K, Hlustik P. Effect of entacapone on plasma homocysteine levels in Parkinson’s disease patients. Neurol Sci. 2010;31(5):565–9.
Nissinen E, Nissinen H, Larjonmaa H, Vaananen A, Helkamaa T, Reenila I, et al. The COMT inhibitor, entacapone, reduces levodopa-induced elevations in plasma homocysteine in healthy adult rats. J Neural Transm. 2005;112(9):1213–21.
Valkovic P, Benetin J, Blazicek P, Valkovicova L, Gmitterova K, Kukumberg P. Reduced plasma homocysteine levels in levodopa/entacapone treated Parkinson patients. Parkinsonism Relat Disord. 2005;11(4):253–6.
Zoccolella S, Iliceto G, de Mari M, Livrea P, Lamberti P. Management of l-Dopa related hyperhomocysteinemia: catechol-O-methyltransferase (COMT) inhibitors or B vitamins? Results from a review. Clin Chem Lab Med. 2007;45(12):1607–13.
Zoccolella S, Lamberti P, Armenise E, de Mari M, Lamberti SV, Mastronardi R, et al. Plasma homocysteine levels in Parkinson’s disease: role of antiparkinsonian medications. Parkinsonism Relat Disord. 2005;11(2):131–3.
Bartl J, Müller T, Grunblatt E, Gerlach M, Riederer P. Chronic monoamine oxidase-B inhibitor treatment blocks monoamine oxidase-A enzyme activity. J Neural Transm. 2014;121(4):379–83.
Przuntek H, Conrad B, Dichgans J, Kraus PH, Krauseneck P, Pergande G, et al. SELEDO: a 5-year long-term trial on the effect of selegiline in early Parkinsonian patients treated with levodopa. Eur J Neurol. 1999;6(2):141–50.
Lyytinen J, Kaakkola S, Ahtila S, Tuomainen P, Teravainen H. Simultaneous MAO-B and COMT inhibition in l-Dopa-treated patients with Parkinson’s disease. Mov Disord. 1997;12(4):497–505.
Müller T, Kuhn W, Przuntek H. Therapy with central active catechol-O-methyltransferase (COMT)-inhibitors: is addition of monoamine oxidase (MAO)-inhibitors necessary to slow progress of neurodegenerative disorders? J Neural Transm Gen Sect. 1993;92(2–3):187–95.
Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32(5):1011–20.
Müller T. Entacapone. Expert Opin Drug Metab Toxicol. 2010;6(8):983–93.
Brooks DJ, Sagar H. Entacapone is beneficial in both fluctuating and non-fluctuating patients with Parkinson’s disease: a randomised, placebo controlled, double blind, six month study. J Neurol Neurosurg Psychiatry. 2003;74(8):1071–9.
Brooks DJ, Agid Y, Eggert K, Widner H, Ostergaard K, Holopainen A. Treatment of end-of-dose wearing-off in Parkinson’s disease: stalevo (levodopa/carbidopa/entacapone) and levodopa/DDCI given in combination with Comtess/Comtan (entacapone) provide equivalent improvements in symptom control superior to that of traditional levodopa/DDCI treatment. Eur Neurol. 2005;53(4):197–202.
Kieburtz K, Hubble J. Benefits of COMT inhibitors in levodopa-treated parkinsonian patients: results of clinical trials. Neurology. 2000;55(11 Suppl 4):S42–5.
Olanow CW, Kieburtz K, Stern M, Watts R, Langston JW, Guarnieri M, et al. Double-blind, placebo-controlled study of entacapone in levodopa-treated patients with stable Parkinson disease. Arch Neurol. 2004;61(10):1563–8.
Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M. Efficacy and safety of entacapone in Parkinson’s disease patients with suboptimal levodopa response: a 6-month randomized placebo-controlled double-blind study in Germany and Austria (Celomen study). Acta Neurol Scand. 2002;105(4):245–55.
Rinne UK, Larsen JP, Siden A, Worm-Petersen J. Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Nomecomt Study Group. Neurology. 1998;51(5):1309–14.
Ruottinen HM, Rinne UK. A double-blind pharmacokinetic and clinical dose-response study of entacapone as an adjuvant to levodopa therapy in advanced Parkinson’s disease. Clin Neuropharmacol. 1996;19(4):283–96.
Ruottinen HM, Rinne UK. Effect of one month’s treatment with peripherally acting catechol-O-methyltransferase inhibitor, entacapone, on pharmacokinetics and motor response to levodopa in advanced parkinsonian patients. Clin Neuropharmacol. 1996;19(3):222–33.
Ruottinen HM, Rinne UK. Entacapone prolongs levodopa response in a one month double blind study in parkinsonian patients with levodopa related fluctuations. J Neurol Neurosurg Psychiatry. 1996;60(1):36–40.
Hauser RA. Levodopa/carbidopa/entacapone (Stalevo). Neurology. 2004;62(1 Suppl 1):S64–71.
Koller W, Guarnieri M, Hubble J, Rabinowicz AL, Silver D. An open-label evaluation of the tolerability and safety of Stalevo (carbidopa, levodopa and entacapone) in Parkinson’s disease patients experiencing wearing-off. J Neural Transm. 2005;112(2):221–30.
Myllyla V, Haapaniemi T, Kaakkola S, Kinnunen E, Hartikainen P, Nuutinen J, et al. Patient satisfaction with switching to Stalevo: an open-label evaluation in PD patients experiencing wearing-off (Simcom Study). Acta Neurol Scand. 2006;114(3):181–6.
Seeberger LC, Hauser RA. Levodopa/carbidopa/entacapone in Parkinson’s disease. Expert Rev Neurother. 2009;9(7):929–40.
Sethi KD, Hauser RA, Isaacson SH, McClain T. Levodopa/carbidopa/entacapone 200/50/200 mg (Stalevo 200) in the treatment of Parkinson’s disease: a case series. Cases J. 2009;2:7134.
Hauser RA, Molho E, Shale H, Pedder S, Dorflinger EE. A pilot evaluation of the tolerability, safety, and efficacy of tolcapone alone and in combination with oral selegiline in untreated Parkinson’s disease patients. Tolcapone De Novo Study Group. Mov Disord. 1998;13(4):643–7.
Entacapone to Tolcapone Switch Study Investigators. Entacapone to tolcapone switch: multicenter double-blind, randomized, active-controlled trial in advanced Parkinson’s disease. Mov Disord. 2007;22(1):14–9.
Ries V, Selzer R, Eichhorn T, Oertel WH, Eggert K. Replacing a dopamine agonist by the COMT-inhibitor tolcapone as an adjunct to l-dopa in the treatment of Parkinson’s disease: a randomized, multicenter, open-label, parallel-group study. Clin Neuropharmacol. 2010;33(3):142–50.
Inzelberg R, Carasso RL, Schechtman E, Nisipeanu P. A comparison of dopamine agonists and catechol-O-methyltransferase inhibitors in Parkinson’s disease. Clin Neuropharmacol. 2000;23(5):262–6.
Koller W, Lees A, Doder M, Hely M. Randomized trial of tolcapone versus pergolide as add-on to levodopa therapy in Parkinson’s disease patients with motor fluctuations. Mov Disord. 2001;16(5):858–66.
Agid Y, Destee A, Durif F, Montastruc JL, Pollak P. Tolcapone, bromocriptine, and Parkinson’s disease. French Tolcapone Study Group. Lancet. 1997;350(9079):712–3.
Martignoni E, Cosentino M, Ferrari M, Porta G, Mattarucchi E, Marino F, et al. Two patients with COMT inhibitor-induced hepatic dysfunction and UGT1A9 genetic polymorphism. Neurology. 2005;65(11):1820–2.
Goetze O, Nikodem AB, Wiezcorek J, Banasch M, Przuntek H, Müller T, et al. Predictors of gastric emptying in Parkinson’s disease. Neurogastroenterol Motil. 2006;18(5):369–75.
Nyholm D, Johansson A, Lennernas H, Askmark H. Levodopa infusion combined with entacapone or tolcapone in Parkinson disease: a pilot trial. Eur J Neurol. 2012;19(6):820–6.
Dingemanse J, Jorga KM, Schmitt M, Gieschke R, Fotteler B, Zurcher G, et al. Integrated pharmacokinetics and pharmacodynamics of the novel catechol-O-methyltransferase inhibitor tolcapone during first administration to humans. Clin Pharmacol Ther. 1995;57(5):508–17.
Kaakkola S, Gordin A, Mannisto PT. General properties and clinical possibilities of new selective inhibitors of catechol O-methyltransferase. Gen Pharmacol. 1994;25(5):813–24.
Maltete D, Cottard AM, Mihout B, Costentin J. Erythrocytes catechol-O-methyl transferase activity is up-regulated after a 3-month treatment by entacapone in parkinsonian patients. Clin Neuropharmacol. 2011;34(1):21–3.
Tuomainen P, Reenila I, Mannisto PT. Validation of assay of catechol-O-methyltransferase activity in human erythrocytes. J Pharm Biomed Anal. 1996;14(5):515–23.
Goncalves D, Alves G, Fortuna A, Soares-da-Silva P, Falcao A. An HPLC-DAD method for the simultaneous quantification of opicapone (BIA 9-1067) and its active metabolite in human plasma. Analyst. 2013;138(8):2463–9.
Goncalves D, Alves G, Soares-da-Silva P, Falcao A. Bioanalytical chromatographic methods for the determination of catechol-O-methyltransferase inhibitors in rodents and human samples: a review. Anal Chim Acta. 2012;710:17–32.
Kiss LE, Ferreira HS, Torrao L, Bonifacio MJ, Palma PN, Soares-da-Silva P, et al. Discovery of a long-acting, peripherally selective inhibitor of catechol-O-methyltransferase. J Med Chem. 2010;53(8):3396–411.
Palma PN, Bonifacio MJ, Loureiro AI, Soares-da-Silva P. Computation of the binding affinities of catechol-O-methyltransferase inhibitors: multisubstate relative free energy calculations. J Comput Chem. 2012;33(9):970–86.
Almeida L, Rocha JF, Falcao A, Palma PN, Loureiro AI, Pinto R, et al. Pharmacokinetics, pharmacodynamics and tolerability of opicapone, a novel catechol-O-methyltransferase inhibitor, in healthy subjects: prediction of slow enzyme-inhibitor complex dissociation of a short-living and very long-acting inhibitor. Clin Pharmacokinet. 2013;52(2):139–51.
Bonifacio MJ, Sutcliffe JS, Torrao L, Wright LC, Soares-da-Silva P. Brain and peripheral pharmacokinetics of levodopa in the cynomolgus monkey following administration of opicapone, a third generation nitrocatechol COMT inhibitor. Neuropharmacology. 2014;77:334–41.
Rocha JF, Almeida L, Falcao A, Palma PN, Loureiro AI, Pinto R, et al. Opicapone: a short lived and very long acting novel catechol-O-methyltransferase inhibitor following multiple dose administration in healthy subjects. Br J Clin Pharmacol. 2013;76(5):763–75.
Nunes T, Rocha JF, Pinto R, Machado R, Wright LC, Falcao A, et al. Pharmacokinetics, pharmacodynamics and tolerability of opicapone, a novel COMT inhibitor, during first administration to healthy male subjects [abstract]. Parkinsonism Relat Disord. 2012;18S2, S81–S159.
Rocha JF, Nunes T, Vaz-da-Silva M, Machado R, Wright LC, Falcao A, et al. Pharmacokinetics, pharmacodynmics and tolerability of opicapone, a novel COMT inhibitor, during multiple dose rise regimen in healthy male subjects [abstract]. Parkinsonism Relat Disord. 2013;18S2, S81–S159.
Feirreira JJ, Rocha JF, Falcao A, Pinto R, Nunes T. Effect of opicapone multiple-dose regimens on levodopa pharmacokinetics, motor response, and erythrocyte-COMT activity in Parkinson’s patients co-administered with levodopa/dopa-decarboxylase inhibitor [abstract]. J Neurol Sci. 2013;333:e109–51.
Lees AJ, Ferreira JJ, Costa R, Rocha JF, Oliveira C, Lopes N. Efficacy and safety of opicapone, a new COMT-inhibitor, for the treatment of motor fluctuations in Parkinson’s Disease patients: BIPARK-II study. J Neurol Sci. 2013;333:e109–51.
Grosset D. Therapy adherence issues in Parkinson’s disease. J Neurol Sci. 2010;289(1–2):115–8.
Richy FF, Pietri G, Moran KA, Senior E, Makaroff LE. Compliance with pharmacotherapy and direct healthcare costs in patients with Parkinson’s disease: a retrospective claims database analysis. Appl Health Econ Health Policy. 2013;11(4):395–406.
Disclosures
Professor Dr. Thomas Müller has performed studies, served on advisory boards and held lectures for Roche, Novartis, Orion and MEDA in the past 20 years. This manuscript was not funded by any of these companies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, T. Catechol-O-Methyltransferase Inhibitors in Parkinson’s Disease. Drugs 75, 157–174 (2015). https://doi.org/10.1007/s40265-014-0343-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0343-0