Abstract
Background and Objective
The number of reports on suspected drug-induced memory impairment submitted to the US Food and Drug Administration increased 30-fold from 2000 to 2022. Drugs are the most common cause of reversible dementia. However, there is very little research on drug-induced cognitive impairment. The aim of this study was to investigate if and how an assessment of cognitive safety was included in recent, registered, controlled, clinical drug trials.
Methods
The clinical trials registry (www.clinicaltrials.gov) was searched for randomized controlled clinical trials with available study protocols. After excluding irrelevant trials such as surgical procedures, local or short-term treatment, and dietary supplements, 803 trials were included in this study. The protocols were manually reviewed for information on if, and how, cognitive safety had been assessed. Trial drugs were categorized into those targeting the central nervous system or not, as well as older and newer drugs. Methods used for the assessment of cognitive function were categorized into questionnaires, screening instruments, and neuropsychological tests. If the trial results were published, we examined whether the publication contained any data on cognitive safety that had emerged from the trial.
Results
The start dates of the screened trials ranged from 31 July, 2009, to 4 April, 2021. Out of the 803 trials, 52 (6.5%) actively assessed cognitive safety. The remaining trials relied solely on spontaneous reporting. Of 429 trials studying a new drug, 32 (7.5%) actively assessed cognitive safety. One hundred and fifty-eight trials examined drugs intended to, or known to have, pharmacological effects on the central nervous system. Of these, 21 (13.5%) assessed cognitive safety. Most of the trials that assessed cognitive safety used either crude screening tools or questionnaires.
Conclusions
Cognitive safety is largely ignored by recent controlled clinical trials. This applies even to trials assessing new drugs and trials assessing central nervous system drugs. There is an urgent need for drug manufacturers, regulatory authorities, and the medical profession to address the cognitive safety of drugs.
Similar content being viewed by others
Only 6.5% of 803 recent clinical drug trials actively assessed cognitive safety. |
Most of the few trials that actively assessed cognitive safety used inappropriate instruments such as questionnaires or crude screening tools. |
When cognitive impairment was found (i.e., as reported on clinicaltrials.gov), it was not always included in the trials's publication or in the drug's prescribing information. |
1 Introduction
Almost 40 years ago, in 1986, it was found that 10% of dementia diagnoses were caused by medication [1]. A follow-up study in 1987 confirmed this finding [2]. A meta-analysis on the causes of dementia concluded that drugs were the most common cause of reversible dementia, accounting for 28.2% of the cases [3]. Given the ever-increasing rate of drug prescription, this number may currently be considerably higher [4]. Adverse drug reaction databases like the FDA Adverse Event Reporting System or the European Medicines Agency’s Eudravigilance contain thousands of reports on suspected adverse cognitive drug reactions. The number of reports on suspected drug-induced memory impairment rose from 381 in the year 2000 to 11,724 in 2022 (FDA Adverse Event Reporting System) [5, 6]. However, there is very little research on drug-induced cognitive impairment (DICI). According to PubMed statistics, there are on average 14 publications per year on “drug-induced dementia” (1968–2022) and 11 per year on “drug-induced cognitive impairment” (1976–2022), without any visible trend indicating increased numbers in the past 25 years [7, 8]. Neither the World Health Organization nor recent public health research mentions drugs as one of the known risk factors for dementia [9, 10].
A large variety of drugs, including those whose primary target is outside the central nervous system (CNS), may impair cognitive function [2, 11]. Probably the best-known example of this are anticholinergic drugs to treat urinary incontinence, but also other frequently prescribed drug classes (e.g., glucocorticoids, statins, non-steroidal anti-inflammatory drugs, or proton pump inhibitors) have a demonstrated potential to impair cognition [12,13,14,15,16]. Drug-induced cognitive impairment ranges from subtle subclinical changes to delirium and dementia, and it is important to note that DICI may affect elderly individuals as well as all other age groups. However, systematic studies on DICI are scarce and often contradictive [17]. Hence, there is a paucity of reliable and recent epidemiological as well as drug-specific data.
A recent meta-analysis found that the number of people living with dementia approximately doubles for every 5 years of age [18]. Because of a growing and aging world population, a large increase in the number of individuals with dementia must be expected in the future. A rise from 57 million cases globally in 2019 to 153 million cases in 2050 has been predicted [19]. As long as there are no effective treatments, emphasis should be placed on efforts to address risk factors. One such risk factor is drug treatment, especially polypharmacy.
Given the above, it should be a priority in clinical drug trials to assess whether the study drug has the potential to induce or worsen cognitive impairment. The aim of this study was to investigate if and how an assessment of cognitive safety was included in recent, registered, controlled, clinical drug trials.
2 Methods
On 6 January, 2023, www.clinicaltrials.gov was accessed and a search was performed with the following 11 filters: (1) randomized controlled trial; (2) not yet recruiting; (3) recruiting; (4) active, not recruiting; (5) completed; (6) phase 2; (7) phase 3; (8) phase 4; (9) interventional; (10) with results; and (11) study protocol. These filters were chosen to maximize the number of relevant hits. The search yielded 1705 clinical trials. Their entries were downloaded in.csv format and imported to Microsoft® Excel® for Microsoft 365 (version 2301).
All trials were manually screened for relevance. The following types of trials were categorized as not relevant for this study: vaccines, vitamins, nutritional supplements, alternative medicines, traditional Chinese medicines, parenteral nutrition, enzymes, surgical or radiological procedures, drugs intended to or hypothesized to improve cognitive function, and most endogenous compounds including their synthetic analogs and derivatives such as stem cells, convalescent plasma, or hormones. Trials involving treatment with glucocorticoids or interferons were not excluded because it has long been known that therapeutic use of these agents can cause cognitive impairment [12, 20, 21]. Trials examining local or topical treatment were excluded.
Although cognitive adverse drug reactions may manifest after only one dose (e.g., sedatives, central analgesics), trials examining short-term treatment (defined as any treatment given or lasting for a maximum of 7 days) were excluded from this study because transient short-term cognitive impairment may be judged as acceptable and, hence, not be actively assessed. However, trials examining repeated cycles of chemotherapy were included as it is well known that chemotherapy may result in sustained cognitive impairment even several months after the last cycle [22]. Trials examining life-sustaining treatment in critically ill patients were excluded, as were trials in children aged less than 1 year.
The remaining trials were categorized as relevant for this study and further categorized into drugs primarily targeting the CNS or not. A drug was considered a CNS drug if it was already approved for a CNS indication, if the main outcome measure in the trial was CNS related, or if the drug has known significant effects on the CNS. Trial drugs were categorized as new drugs if they were not yet approved in one of the major drug markets (USA, European Union, or Japan) at the start date of the trial, or if they were approved less than 1 year earlier.
The Study Plan (“How is the study designed?” and “What is the study measuring?”) of each trial was reviewed at www.clinicaltrials.gov. The study protocol of each trial was then manually examined for information indicating that cognitive safety had been assessed during or at the end of the trial. If cognitive function had been assessed as a safety parameter, the available information was further reviewed for information on which instruments were used for such assessment. The last step was to gather information on the results of the cognitive safety assessment (from each trial’s “Results” entry on www.clinicaltrials.gov) and, if the trial was published, whether these results were included in the publication or not.
3 Results
3.1 Main Findings
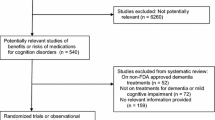
The main findings are summarized in Fig. 1. The start dates of the screened trials ranged from 31 July, 2009 to 4 April, 2021. Out of the initial 1705 trials, 630 were categorized as not relevant for this study, 79 examined cognition-improving drugs, and 187 studied short-term treatment. These trials were excluded. Of the remaining 809 trials, three had study protocols that lacked relevant information on safety assessments. One study protocol’s safety section was extensively censored, and for two trials the study protocol was not available. Of the 803 trials that could be evaluated, 352 were phase II trials, 388 were phase III, and 64 were phase IV. New drugs were studied by 429 of the 803 trials. Drugs affecting the CNS were studied by 158 trials.
Fifty-two out of the 803 trials (6.5%) actively assessed cognitive safety. The remaining trials relied solely on spontaneous reporting from study participants, their relatives, or caregivers. Of the 429 trials studying a new drug, three could not be evaluated because of a lack of relevant information. Of the remaining 426 trials, 32 (7.5%) actively assessed cognitive safety.
One hundred and fifty-eight trials examined drugs intended to or known to have pharmacological effects on the CNS. Three of them were not assessable (no study protocol: 2, extensive censoring: 1). Of the remaining 155 trials, 21 (13.5%) assessed cognitive safety.
In total, 37 trials used questionnaires or screening tools. Sixteen of them reported cognitive adverse events on www.clinicaltrials.gov. Thirteen trials used specified neuropsychological tests addressing distinct cognitive domains. Six of them reported cognitive adverse events. Two trials used unspecified neuropsychological tests. One of them reported cognitive adverse events. The details of the 52 trials that actively assessed cognitive safety are given in the Table 1 of the Electronic Supplementary Material (ESM).
3.2 Observations
In most trials, the safety assessment consisted of monitoring vital signs (heart rate, respiration, blood pressure), physical examination, laboratory assessments, and most often an electrocardiogram. In some trials, a neurological examination was added. However, a considerable number of trials assessed only vital signs or did not perform any safety assessment at all. These study protocols often referred to the fact that the study drug was already approved. A common phrase in such study protocols would read “[drug name] is an FDA-approved drug that has been in clinical use for many years and has a well-known adverse effect profile.”
Some study protocols (e.g., NCT02938520, NCT02666664, or NCT02706951) stated that “neurocognitive events will be identified and evaluated by routine safety monitoring” and specified how the severity of such cognitive adverse events should be graded. However, they did not actively assess cognitive safety but relied on spontaneous reporting by participants, their relatives, or caregivers. This includes trials examining CNS-active drugs such as cannabinoids (e.g., NCT02318602).
Several study protocols (e.g., NCT01636934, NCT03434041, NCT02677896, NCT02101853, NCT02249091, or NCT03545191) explicitly stated that the trial drug, the drug class it belongs to, or other drugs used in the trial (i.e., comparator drugs) had demonstrated their potential to evoke DICI but did not assess cognitive safety. This includes trials studying not yet approved drugs and drugs targeting the CNS. Some study protocols (e.g., NCT03156621) defined neurocognitive events as adverse events of special interest but did not actively screen for them.
A large number of trials (e.g., NCT02696785) actively assessed quality-of-life parameters including mental health or mental well-being by using questionnaires. While these questionnaires included, for example, depressive symptoms or signs of suicidality, they did not include cognitive function.
4 Discussion
Most trials reviewed in this study relied on spontaneous reporting of DICI by participants, relatives, or caregivers instead of actively assessing cognitive safety. However, DICI is not synonymous with manifest dementia. It may remain mild yet measurable with appropriate neuropsychological tests. Drug-induced cognitive impairment is not always recognized by patients or their relatives and even if cognitive impairment is noticed, it may be interpreted as symptoms of disorders such as depression or Alzheimer’s disease [23]. This implies a considerable risk of non-detection and, therefore, under-reporting in clinical trials. The risk of under-reporting is further increased as patients wishing to receive the experimental treatment may be afraid of being excluded from the study if they report adverse drug reactions. Hence, it is not sufficient to rely on self-reporting of DICI.
There are obvious reasons why DICI should be recognized and evaluated in clinical trials. For one, DICI accelerates cognitive aging [15, 24,25,26]. It may lead to a new diagnosis in addition to the patient’s original condition, thereby increasing the number of drugs prescribed to this patient, which in turn may increase the risk of DICI. Second, DICI is not always so severe that it is noticed by mere observation.
However, even subtle changes in cognition and behavior may have a significant impact on academic achievement, work, or social functioning. Such subtle changes are rarely detected by using crude screening tools such as the Mini-Mental State Examination (MMSE) or the Montreal Cognitive Assessment [27]. Screening tools or questionnaires such as those developed by the European Organisation for the Research and Treatment of Cancer have low sensitivity for detecting non-serious cognitive adverse effects [27,28,29,30]. For example, only three out of the maximum achievable 30 points in the MMSE account for learning and long-term retrieval. This implies that if a person with apparent memory problems has little difficulty with the remaining items in the MMSE, they may achieve a total score of 27 points. This would be interpreted as normal cognitive function. A deterioration in memory function in an otherwise highly functioning, intelligent and/or younger person may thus remain undetected.
This is further illustrated by the fact that several trials that actively assessed cognitive safety did not detect DICI even when the trial drug’s potential to induce them was declared in its prescribing information (see Table 1 of the ESM). This may have been due to coincidence or the study design but in many cases, it appears more likely to be a consequence of using questionnaires, tests with low sensitivity (e.g., the MMSE), or tests that are not suitable to detect DICI. For example, tests focusing on behavior (e.g., Behavior Rating Inventory of Executive Function Preschool Version) or intelligence (e.g., Wechsler Adult Intelligence Scale, Third Edition) are poorly suited to detect impaired cognitive function. By contrast, some trials did report cognitive adverse events with frequencies >5% on www.clinicaltrials.gov, but these are neither reported in the formal publication nor in the respective prescribing information. Examples of the latter are NCT01982955 (tefotinib) and NCT02308020 (abemaciclib).
Seven of the 15 trials that used neuropsychological tests reported cognitive adverse events. Notably, two of the four trials with esketamine and one trial with an anti-seizure medication (lacosamide) did not. Their prescribing information declares cognitive adverse effects. However, the lacosamide trial used the Behavior Rating Inventory of Executive Function Preschool Version. The esketamine trials used a non-specified test battery that possibly was inappropriately composed.
It may be argued that it is not imperative to evaluate the cognitive safety of a drug already approved and clinically used for years, perhaps even decades. This is not a valid argument because, not least according to the findings in the present study, the cognitive safety of most drugs has very likely never been appropriately examined.
It might be reasonable to expect that new unapproved drugs or drugs marketed for no more than a year would be more thoroughly assessed regarding cognitive safety than older drugs. Our results do not support this notion, even if it is well known that previously unreported adverse drug reactions may keep emerging for years after a drug’s first approval [31].
Given the absence of appropriate assessment of cognitive safety in clinical trials and the focus on manifest dementia in post-marketing DICI research, it is not surprising that meta-analyses reviewing the evidence for DICI of specific drugs find inconclusive, weak, or no such evidence at all [32, 33]. As a side note, most trials in this study used a public reporting threshold of 5% for non-serious adverse events, meaning that even if cognitive adverse events should occur in as many as 49 out of 1000 patients, they would not be publicly reported.
Some publications stated that the severity grading of adverse events was performed by the investigator, and only reported “serious or severe common adverse events”. An example is the shared benefit-risk assessment article of the esketamine trials (see Table 1 of the ESM) [34]. This publication does not report any cognitive adverse events at all even if a confusional state and mental impairment were observed in 5.9% and 5.2% in two of the trials (according to clinicaltrials.gov). Instead, it states: “There was also no difference between treatment groups in any of the cognitive tests performed during TRANSFORM‐1 or TRANSFORM‐2”. This statement seems to be in contrast to the reported adverse events of NCT02417064 (TRANSFORM-1) on www.clinicaltrials.gov where mental impairment was observed in 5.22% of the 56-mg 2 × /week esketamine arm and in 0.88% of the placebo arm. Mental impairment is used here as a Medical Dictionary for Regulatory Activities term, meaning that it is a subclass of Cognitive and attention disorders [35].
Most of the observed cognitive adverse events are not trivial (see Table 1 of the ESM). For instance, aphasia, amnesia, and memory impairment can have a devastating impact on a person’s professional and social life. If not recognized as DICI, they may be misinterpreted as symptoms of an underlying disease and the patient may be given a new diagnosis as well as, presumably, additional drug treatment [20, 36].
Decades ago, it was found that DICI is the cause of 10% of dementia cases [1, 2]. In the absence of more recent epidemiological data, the current prevalence of drug-induced dementia and other forms of DICI is unknown. Cognitive impairment and dementia can have enormous consequences for those who are affected, and for their families. In addition, they strain public health systems and, through sick leave or early retirement, deprive enterprises and national economies of individuals with professional knowledge and experience [37, 38]. Regulatory agencies should demand active cognitive monitoring in all clinical drug trials. We recommend that each cognitive domain be assessed. Ideally, the tests should be selected by an experienced neuropsychologist according to the type of the expected possible cognitive effects. This should be mandatory for drugs known to, intended to, or suspected to affect the CNS, and desirable for all other drugs. The minimum should be a cognitive screening tool of the more domain-specific type, selected according to the study population, disease, and drug studied, such as EpiTrack®, the Rowland Universal Dementia Assessment Scale, or the Mini-Cog®.
5 Conclusions
Almost all trials reviewed in this study actively assessed physical drug safety including electrocardiogram and laboratory values. By contrast, cognitive drug safety is largely ignored. This applies even to trials assessing new drugs and trials assessing drugs targeting the CNS. Only 6.5% of the trials reviewed in this study actively assessed cognitive safety. Most of them used screening tools or questionnaires. In an era of increasing prevalence of dementia and a growing number of reports on suspected DICI, these are deplorable findings. Cardiac safety in most clinical trials is assessed with an electrocardiogram and not a with a questionnaire. Likewise, cognitive safety should be assessed with appropriate neuropsychological instruments. There is an urgent need for drug manufacturers, regulatory authorities, and the medical profession to address the cognitive safety of drugs.
References
Larson EB, Reifler BV, Sumi SM, Canfield CG, Chinn NM. Features of potentially reversible dementia in elderly outpatients. West J Med. 1986;145(4):488–92.
Larson EB, Kukull WA, Buchner D, Reifler BV. Adverse drug reactions associated with global cognitive impairment in elderly persons. Ann Intern Med. 1987;107(2):169–73.
Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med. 1988;109(6):476–86.
National Center for Health Statistics. National Health and Nutrition Examination Survey: prescription drug use in the past 30 days, by sex, race and Hispanic origin, and age: United States, selected years 1988–1994 through 2015–2018—Con. 2019. https://www.cdc.gov/nchs/data/hus/2019/039-508.pdf. Accessed 16 Sep 2023.
FDA Adverse Event Reporting System (FAERS) Public Dashboard. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard. Accessed 16 Sep 2023.
EudraVigilance: European database of suspected drug reaction reports. https://www.adrreports.eu. Accessed 16 Sep 2023.
PubMed. PubMed search: “Drug-induced cognitive impairment”. Results by year. https://pubmed.ncbi.nlm.nih.gov/?term=drug-induced+cognitive+impairment&filter=years.1976-2022&timeline=expanded&sort=date. Accessed 21 Feb 2023.
PubMed. PubMed search “Drug-induced dementia”. Results by year. https://pubmed.ncbi.nlm.nih.gov/?term=drug-induced+dementia&filter=years.1968-2022&sort=date. Accessed 21 Feb 2023.
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46.
WHO. Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed 16 Sep 2023.
Starr JM, Whalley LJ. Drug-induced dementia: incidence, management and prevention. Drug Saf. 1994;11(5):310–7.
Varney NR, Alexander B, MacIndoe JH. Reversible steroid dementia in patients without steroid psychosis. Am J Psychiatry. 1984;141(3):369–72.
Tan B, Rosenfeldt F, Ou R, Stough C. Evidence and mechanisms for statin-induced cognitive decline. Expert Rev Clin Pharmacol. 2019;27:1–10.
Sonnen JA, Larson EB, Walker RL, Haneuse S, Crane PK, Gray SL, et al. Nonsteroidal anti-inflammatory drugs are associated with increased neuritic plaques. Neurology. 2010;75(13):1203–10.
Akter S, Hassan MR, Shahriar M, Akter N, Abbas MG, Bhuiyan MA. Cognitive impact after short-term exposure to different proton pump inhibitors: assessment using CANTAB software. Alzheimers Res Ther. 2015;27(7):79.
Northuis C, Bell E, Lutsey P, George KM, Gottesman RF, Mosley TH, et al. Cumulative use of proton pump inhibitors and risk of dementia: the Atherosclerosis Risk in Communities Study. Neurology. 2023;101(18):e1771–8.
Jamshidnejad-Tosaramandani T, Kashanian S, Al-Sabri MH, Krocianova D, Clemensson LE, Gentreau M, et al. Statins and cognition: modifying factors and possible underlying mechanisms. Front Aging Neurosci. 2022;14: 968039.
Cao Q, Tan CC, Xu W, Hu H, Cao XP, Dong Q, et al. The prevalence of dementia: a systematic review and meta-analysis. J Alzheimers Dis. 2020;73(3):1157–66.
Global Burden of Disease 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–25.
Sacks O, Shulman M. Steroid dementia: an overlooked diagnosis? Neurology. 2005;64(4):707–9.
Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser P. Mood and cognitive side effects of interferon-alpha therapy. Semin Oncol. 1998;25(1 Suppl. 1):39–47.
Ibrahim EY, Domenicano I, Nyhan K, Elfil M, Mougalian SS, Cartmel B, et al. Cognitive effects and depression associated with taxane-based chemotherapy in breast cancer survivors: a meta-analysis. Front Oncol. 2021;11: 642382.
Evans MA, Golomb BA. Statin-associated adverse cognitive effects: survey results from 171 patients. Pharmacotherapy. 2009;29(7):800–11.
Kumar R, Kumar A, Nordberg A, Langstrom B, Darreh-Shori T. Proton pump inhibitors act with unprecedented potencies as inhibitors of the acetylcholine biosynthesizing enzyme: a plausible missing link for their association with incidence of dementia. Alzheimers Dement. 2020;16(7):1031–42.
Lopez-Alvarez J, Sevilla-Llewellyn-Jones J, Aguera-Ortiz L. Anticholinergic drugs in geriatric psychopharmacology. Front Neurosci. 2019;13:1309.
Marvanova M. Drug-induced cognitive impairment: effect of cardiovascular agents. Ment Health Clin. 2016;6(4):201–6.
Roebuck-Spencer TM, Glen T, Puente AE, Denney RL, Ruff RM, Hostetter G, et al. Cognitive screening tests versus comprehensive neuropsychological test batteries: a National Academy of Neuropsychology Education Paperdagger. Arch Clin Neuropsychol. 2017;32(4):491–8.
Herreen D, Zajac IT. The reliability and validity of a self-report measure of cognitive abilities in older adults: more personality than cognitive function. J Intell. 2017;6(1):1.
Weinstein AM, Gujral S, Butters MA, Bowie CR, Fischer CE, Flint AJ, et al. Diagnostic precision in the detection of mild cognitive impairment: a comparison of two approaches. Am J Geriatr Psychiatry. 2022;30(1):54–64.
Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, et al. Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers. 2021;7(1):47.
Downing NS, Shah ND, Aminawung JA, Pease AM, Zeitoun JD, Krumholz HM, et al. Postmarket safety events among novel therapeutics approved by the US Food and Drug Administration between 2001 and 2010. JAMA. 2017;317(18):1854–63.
Ott BR, Daiello LA, Dahabreh IJ, Springate BA, Bixby K, Murali M, et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2015;30(3):348–58.
Ahn N, Nolde M, Krause E, Guntner F, Gunter A, Tauscher M, et al. Do proton pump inhibitors increase the risk of dementia? A systematic review, meta-analysis and bias analysis. Br J Clin Pharmacol. 2023;89(2):602–16.
Katz EG, Hough D, Doherty T, Lane R, Singh J, Levitan B. Benefit-risk assessment of esketamine nasal spray vs. placebo in treatment-resistant depression. Clin Pharmacol Ther. 2021;109(2):536–46.
National Center for Biomedical Ontology. Bioportal. Medical dictionary for regulatory activities terminology (MedDRA). https://bioportal.bioontology.org/ontologies/MEDDRA. Accessed 12 Sep 2023.
Lindbom U, Taubert B, Fahlqvist MS, Bergens A, Kimland E, Jonsson EW, et al. Reversible dementia-like condition and parkinsonism in an elderly woman. Idiosyncratic adverse effects connected to 11 years of antiepileptic medication. Lakartidningen. 2009;106(12):863–5.
Velandia PP, Miller-Petrie MK, Chen C, Chakrabarti S, Chapin A, Hay S, et al. Global and regional spending on dementia care from 2000–2019 and expected future health spending scenarios from 2020–2050: an economic modelling exercise. EClinicalMedicine. 2022;45: 101337.
UK Government. Press release: businesses risk losing billions unless they adapt: report reveals the future cost of dementia. https://www.gov.uk/government/news/businesses-risk-losing-billions-unless-they-adapt-report-reveals-the-future-cost-of-dementia. Accessed 12 Sep 2023.
Acknowledgements
We thank Robin Morris for proofreading and language editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Lund University.
Conflict of interest
Arne Reimers and Hanna Ljung have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Upon reasonable request, data will be shared with qualified scientific and medical researchers. All requests should be submitted in writing to the corresponding author.
Code availability
Not applicable.
Author contributions
AR study concept; collection, analysis and interpretation of data; drafting/revision of the manuscript. HL analysis and interpretation of data; drafting/revision of the manuscript. Both authors read and approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Reimers, A., Ljung, H. Cognitive Safety is Largely Ignored in Clinical Drug Trials: A Study of Registered Study Protocols. Drug Saf 47, 23–28 (2024). https://doi.org/10.1007/s40264-023-01378-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-023-01378-1