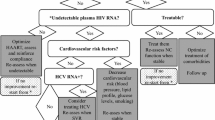
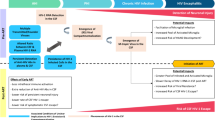
Abstract
HIV-positive patients may be effectively treated with highly active antiretroviral therapy and such a strategy is associated with striking immune recovery and viral load reduction to very low levels. Despite undeniable results, the central nervous system (CNS) is commonly affected during the course of HIV infection, with neurocognitive disorders being as prevalent as 20–50 % of treated subjects. This review discusses the pathophysiology of CNS infection by HIV and the barriers to efficacious control of such a mechanism, including the available data on compartmental drug penetration and on pharmacokinetic/pharmacodynamic relationships. In the reviewed articles, a high variability in drug transfer to the CNS is highlighted with several mechanisms as well as methodological issues potentially influencing the observed results. Nevirapine and zidovudine showed the highest cerebrospinal fluid (CSF) to plasma ratios, although target concentrations are currently unknown for the CNS. The use of the composite CSF concentration effectiveness score has been associated with better virological outcomes (lower HIV RNA) but has been inconsistently associated with neurocognitive outcomes. These findings support the CNS effectiveness of commonly used highly antiretroviral therapies. The use of antiretroviral drugs with increased CSF penetration and/or effectiveness in treating or preventing neurocognitive disorders however needs to be assessed in well-designed prospective studies.
Similar content being viewed by others
References
Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–82.
Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011;24(3):275–83.
Zhou L, Saksena NK. HIV associated neurocognitive disorders. Infect Dis Rep. 2013;5(Suppl 1):e8.
Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–86.
Bonnet F, Amieva H, Marquant F, et al. Cognitive disorders in HIV-infected patients: are they HIV-related? AIDS. 2013;27(3):391–400.
Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev. 2013;254(1):102–13.
Carroll-Anzinger D, Kumar A, Adarichev V, Kashanchi F, Al-Harthi L. Human immunodeficiency virus-restricted replication in astrocytes and the ability of gamma interferon to modulate this restriction are regulated by a downstream effector of the Wnt signaling pathway. J Virol. 2007;81(11):5864–71.
Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–72.
Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–62.
Anthony IC, Bell JE. The neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008;20:15–24.
Kaul M. HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Curr Opin Neurol. 2009;22(3):315–20.
Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91(3):401–15.
Nakagawa S, Castro V, Toborek M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood–brain barrier. J Cell Mol Med. 2012;16(12):2950–7.
Abdulle S, Hagberg L, Gisslén M. Effects of antiretroviral treatment on blood–brain barrier integrity and intrathecal immunoglobulin production in neuroasymptomatic HIV-1-infected patients. HIV Med. 2005;6(3):164–9.
Abdulle S, Hagberg L, Svennerholm B, Fuchs D, Gisslén M. Continuing intrathecal immunoactivation despite two years of effective antiretroviral therapy against HIV-1 infection. AIDS. 2002;16(16):2145–9.
Calcagno A, Alberione MC, Romito A, Imperiale D, Ghisetti V, Audagnotto S, et al. Prevalence and predictors of blood brain barrier damage in the HAART Era. J Neurovirol. Epub 2014 Jun 28.
Shen L, Siliciano RF. Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J Allergy Clin Immunol. 2008;122(1):22–8.
Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. 2014;111(6):2307–12.
Schnell G, Spudich S, Harrington P, Price RW, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog. 2009;5(4):e1000395.
Zhang Y, Wei F, Liang Q, et al. High levels of divergent HIV-1 quasispecies in patients with neurological opportunistic infections in China. J Neurovirol. 2013;19(4):359–66.
Edén A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202(12):1819–25.
Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–8.
Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26(14):1765–74.
Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99.
Heaton R, Franlin D, Woods S, et al. Asymptomatic mild HIV-associated neurocognitive disorder increases risk for future symptomatic decline: a CHARTER longitudinal study [abstract]. 19th Conference on Retroviruses and Opportunistic Infections (CROI); 5–8 Mar 2012; Seattle.
Blackstone K, Moore DJ, Heaton RK, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012;18(1):79–88.
Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol. 2011;17(2):176–83.
Winston A, Arenas-Pinto A, Stöhr W, et al. Neurocognitive function in HIV infected patients on antiretroviral therapy. PLoS One. 2013;8(4):e61949.
Spudich S. HIV and neurocognitive dysfunction. Curr HIV/AIDS Rep. 2013;10(3):235–43. doi:10.1007/s11904-013-0171-y.
Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood–CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82(2):A99–109.
Kumar AM, Borodowsky I, Fernandez B, Gonzalez L, Kumar M. Human immunodeficiency virus type 1 RNA Levels in different regions of human brain: quantification using real-time reverse transcriptase-polymerase chain reaction. J Neurovirol. 2007;13(3):210–24.
Fox E, Bungay PM, Bacher J, et al. Zidovudine concentration in brain extracellular fluid measured by microdialysis: steady-state and transient results in rhesus monkey. J Pharmacol Exp Ther. 2002;301(3):1003–11.
Liu X, Van Natta K, Yeo H, et al. Unbound drug concentration in brain homogenate and cerebral spinal fluid at steady state as a surrogate for unbound concentration in brain interstitial fluid. Drug Metab Dispos. 2009;37(4):787–93.
Blaney SM, Daniel MJ, Harker AJ, Godwin K, Balis FM. Pharmacokinetics of lamivudine and BCH-189 in plasma and cerebrospinal fluid of nonhuman primates. Antimicrob Agents Chemother. 1995;39(12):2779–82.
Caruso A, Alvarez-Sánchez R, Hillebrecht A, et al. PK/PD assessment in CNS drug discovery: prediction of CSF concentration in rodents for P-glycoprotein substrates and application to in vivo potency estimation. Biochem Pharmacol. 2013;85(11):1684–99.
de Lange EC. Utility of CSF in translational neuroscience. J Pharmacokinet Pharmacodyn. 2013;40(3):315–26.
Eisfeld C, Reichelt D, Evers S, Husstedt I. CSF penetration by antiretroviral drugs. CNS Drugs. 2013;27(1):31–55.
Enting RH, Hoetelmans RM, Lange JM, et al. Antiretroviral drugs and the central nervous system. AIDS. 1998;12:1941–55.
Soulas C, Conerly C, Kim WK, et al. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–35.
Minogue AM, Jones RS, Kelly RJ, McDonald CL, Connor TJ, Lynch MA. Age-associated dysregulation of microglial activation is coupled with enhanced blood-brain barrier permeability and pathology in APP/PS1 mice. Neurobiol Aging. 2014;35(6):1442–52.
Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and clinical features of cerebrovascular disease among HIV-infected adults in the Southeastern United States. AIDS Res Hum Retroviruses. 2013;29(7):1068–74.
Singer EJ, Valdes-Sueiras M, Commins DL, Yong W, Carlson M. HIV stroke risk: evidence and implications. Ther Adv Chronic Dis. 2013;4(2):61–70.
Croteau D, Best B, Clifford D, et al. Older age is associated with higher ARV concentrations in CSF in HIV+ individuals [abstract]. 19th Conference on Retroviruses and Opportunistic Infections (CROI); 5–8 Mar 2012; Seattle.
Moss DM, Siccardi M, Back DJ, Owen A. Predicting intestinal absorption of raltegravir using a population-based ADME simulation. J Antimicrob Chemother. 2013;68(7):1627–34.
Marshall DW, Brey RL, Butzin CA, et al. Spectrum of cerebrospinal fluid findings in various stages of human immunodeficiency virus infection. Arch Neurol. 1988;45:954–8.
Petito CK, Cash KS. Blood–brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 1992;32:658–66.
Andersson LM, Hagbwerg L, Fuchs D, Svennerholm B, Gisslen M. Increased blood brain-barrier permeability in neuroasymptomatic HIV-1-infected individuals-correlation with cerebrospinal fluid HIV-1 RNA and neopterin levels. J Neurovirol. 2001;7:542–7.
Marchi N, Betto G, Fazio V, et al. Blood–brain barrier damage and brain penetration of antiepileptic drugs: role of serum proteins and brain edema. Epilepsia. 2009;50(4):664–77.
Yilmaz A, Gisslén M, Spudich S, et al. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS One. 2009;4(9):e6877.
Calcagno A, Bonora S, Simiele M, et al. Tenofovir and emtricitabine cerebrospinal fluid-to-plasma ratios correlate to the extent of blood–brain barrier damage. AIDS. 2011;25(11):1437–9.
Calcagno A, Cusato J, Simiele M, et al. High interpatient variability of raltegravir cerebrospinal fluid concentrations in HIV-positive patients: a pharmacogenetic analysis. J Antimicrob Chemother. 2014;69(1):241–5.
Marzolini C, Mueller R, Li-Blatter X, Battegay M, Seelig A. The brain entry of HIV-1 protease inhibitors is facilitated when used in combination. Mol Pharm. 2013;10(6):2340–9.
Avery LB, Zarr MA, Bakshi RP, Siliciano RF, Hendrix CW. Increasing extracellular protein concentration reduces intracellular antiretroviral drug concentration and antiviral effect. AIDS Res Hum Retroviruses. 2013;29(11):1434–42.
Yilmaz A, Ståhle L, Hagberg L, et al. Cerebrospinal fluid and plasma HIV-1 RNA levels and lopinavir concentrations following lopinavir/ritonavir regimen. Scand J Infect Dis. 2004;36(11–12):823–8.
Croteau D, Best BM, Letendre S, et al. Lower than expected maraviroc concentrations in cerebrospinal fluid exceed the wild-type CC chemokine receptor 5-tropic HIV-1 50 % inhibitory concentration. AIDS. 2012;26(7):890–3.
Croteau D, Rossi SS, Best BM, et al. Darunavir is predominantly unbound to protein in cerebrospinal fluid and concentrations exceed the wild-type HIV-1 median 90 % inhibitory concentration. J Antimicrob Chemother. 2013;68(3):684–9.
Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003;21(3–4):79–96.
Avery LB, Sacktor N, McArthur JC, Hendrix CW. Protein-free efavirenz concentrations in cerebrospinal fluid and blood plasma are equivalent: applying the law of mass action to predict protein-free drug concentration. Antimicrob Agents Chemother. 2013;57(3):1409–14.
Delille CA, Pruett ST, Marconi VC, et al. Effect of protein binding on unbound atazanavir and darunavir cerebrospinal fluid concentrations. J Clin Pharmacol. 2014;54(9):1063–71. doi:10.1002/jcph.298.
Nguyen A, Rossi S, Croteau D, et al. Etravirine in CSF is highly protein bound. J Antimicrob Chemother. 2013;68(5):1161–8.
Stępień KM, Tomaszewski M, Tomaszewska J, Czuczwar SJ. The multidrug transporter P-glycoprotein in pharmacoresistance to antiepileptic drugs. Pharmacol Rep. 2012;64(5):1011–9.
Kannan P, John C, Zoghbi SS, et al. Imaging the function of P-glycoprotein with radiotracers: pharmacokinetics and in vivo applications. Clin Pharmacol Ther. 2009;86(4):368–77.
Bleasby K, Castle JC, Roberts CJ, et al. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica. 2006;36(10–11):963–88.
Hartkoorn RC, Kwan WS, Shallcross V, et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics. 2010;20(2):112–20.
Kaddoumi A, Choi SU, Kinman L, et al. Inhibition of P-glycoprotein activity at the primate blood–brain barrier increases the distribution of nelfinavir into the brain but not into the cerebrospinal fluid. Drug Metab Dispos. 2007;35(9):1459–62.
Calcagno A, Yilmaz A, Cusato J, et al. Determinants of darunavir cerebrospinal fluid concentrations: impact of once-daily dosing and pharmacogenetics. AIDS. 2012;26(12):1529–33.
Zakeri-Milani P, Valizadeh H. Intestinal transporters: enhanced absorption through P-glycoprotein-related drug interactions. Expert Opin Drug Metab Toxicol. 2014;10(6):859-71.
Lepist EI, Phan TK, Roy A, et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother. 2012;56(10):5409–13.
Sánchez Martín A, Cabrera Figueroa S, Cruz Guerrero R, et al. Impact of pharmacogenetics on CNS side effects related to efavirenz. Pharmacogenomics. 2013;14(10):1167–78.
Wyen C, Hendra H, Siccardi M, et al. Cytochrome P450 2B6 (CYP2B6) and constitutive androstane receptor (CAR) polymorphisms are associated with early discontinuation of efavirenz-containing regimens. J Antimicrob Chemother. 2011;66(9):2092–8.
Saitoh A, Sarles E, Capparelli E, et al. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. AIDS. 2007;21(16):2191–9.
Best BM, Letendre SL, Brigid E, et al. Low atazanavir concentrations in cerebrospinal fluid. AIDS. 2009;23(1):83–7.
Capparelli EV, Letendre SL, Ellis RJ, et al. Population pharmacokinetics of abacavir in plasma and cerebrospinal fluid. Antimicrob Agents Chemother. 2005;49(6):2504–6.
Yilmaz A, Watson V, Else L, Gisslèn M. Cerebrospinal fluid maraviroc concentrations in HIV-1 infected patients. AIDS. 2009;23(18):2537–40.
Melica G, Canestri A, Peytavin G, et al. Maraviroc-containing regimen suppresses HIV replication in the cerebrospinal fluid of patients with neurological symptoms. AIDS. 2010;24(13):2130–3.
Tiraboschi JM, Niubo J, Curto J, Podzamczer D. Maraviroc concentrations in cerebrospinal fluid in HIV-infected patients. J Acquir Immune Defic Syndr. 2010;55(5):606–9.
Burger DM, Kraaijeveld CL, Meenhorst PL, et al. Penetration of zidovudine into the cerebrospinal fluid of patients infected with HIV. AIDS. 1993;7:1581–7.
Lane HC, Falloon J, Walker RE, et al. Zidovudine in patients with human immunodeficiency virus (HIV) infection and Kaposi sarcoma. A phase II randomized, placebo-controlled trial. Ann Intern Med. 1989;111:41–50.
Elovaara I, Poutiainen E, Lahdevirta J, et al. Zidovudine reduces intrathecal immunoactivation in patients with early human immunodeficiency virus type 1 infection. Arch Neurol. 1994;51:943–50.
Balis FM, Pizzo PA, Eddy J, et al. Pharmacokinetics of zidovudine administered intravenously and orally in children with human immunodeficiency virus infection. J Pediatr. 1989;114:880–4.
Hagberg L, Andersson M, Chiodi F, et al. Effect of zidovudine on cerebrospinal fluid in patients with HIV infection and acute neurological disease. Scand J Infect Dis. 1991;23:681–5.
Tozzi V, Narciso P, Galgani S, et al. Effects of zidovudine in 30 patients with mild to end-stage AIDS dementia complex. AIDS. 1993;7:683–92.
Sidtis JJ, Gatsonis C, Price RW, et al. Zidovudine treatment of the AIDS dementia complex: results of a placebo-controlled trial. AIDS Clinical Trials Group. Ann Neurol. 1993;33:343–9.
McDowell JA, Chittick GE, Ravitch JR, et al. Pharmacokinetics of [(14)C]abacavir, a human immunodeficiency virus type 1 (HIV-1) reverse transcriptase inhibitor, administered in a single oral dose to HIV-1-infected adults: a mass balance study. Antimicrob Agents Chemother. 1999;43:2855–61.
McDowell JA, Lou Y, Symonds WS, et al. Multiple-dose pharmacokinetics and pharmacodynamics of abacavir alone and in combination with zidovudine in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2000;44:2061–7.
Brew BJ, Halman M, Catalan J, et al. Factors in AIDS dementia complex trial design: results and lessons from the abacavir trial. PLoS Clin Trials. 2007;2:e13.
Antinori A, Perno CF, Giancola ML, et al. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis. 2005;41:1787–93.
Burger DM, Kraayeveld CL, Meenhorst PL, et al. Study on didanosine concentrations in cerebrospinal fluid. Implications for the treatment and prevention of AIDS dementia complex. Pharm World Sci. 1995;17:218–21.
Gisslén M, Norkrans G, Svennerholm B, et al. The effect on human immunodeficiency virus type 1 RNA levels in cerebrospinal fluid after initiation of zidovudine or didanosine. J Infect Dis. 1997;175:434–7.
Best B, Letendre S, Capparelli E, et al. Efavirenz and emtricitabine concentrations consistently exceed wild-type IC50 in cerebrospinal fluid: CHARTER findings [abstract]. 16th Conference on Retroviruses and Opportunistic Infections (CROI); 8–11 Feb 2009; Montreal.
Foudraine NA, Hoetelmans RM, Lange JM, et al. Cerebrospinal-fluid HIV-1 RNA and drug concentrations after treatment with lamivudine plus zidovudine or stavudine. Lancet. 1998;351:1547–51.
Blaschke A, Capparelli E, Ellis R, et al. A population model-based approach for determining lamivudine (3TC) cerebrospinal fluid (CSF) penetration in HIV-infected adults [abstract]. 7th Conference on Retroviruses and Opportunistic Infections (CROI); 30 Jan–2 Feb 2000; San Francisco.
Haworth SJ, Christofalo B, Anderson RD, et al. A single-dose study to assess the penetration of stavudine into human cerebrospinal fluid in adults. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:235–8.
Brady KA, Boston RC, Aldrich JL, et al. Stavudine entry into cerebrospinal fluid after single and multiple doses in patients infected with human immunodeficiency virus. Pharmacotherapy. 2005;25:10–7.
Zhang L, Price R, Aweeka F, et al. Making the most of sparse clinical data by using a predictive-model-based analysis, illustrated with a stavudine pharmacokinetic study. Eur J Pharm Sci. 2001;12:377–85.
Best BM, Letendre SL, Koopmans P, et al. Low cerebrospinal fluid concentrations of the nucleotide HIV reverse transcriptase inhibitor, tenofovir. J Acquir Immune Defic Syndr. 2012;59(4):376–81.
Takasawa K, Terasaki T, Suzuki H, et al. Distributed model analysis of 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine distribution in brain tissue and cerebrospinal fluid. J Pharmacol Exp Ther. 1997;282:1509–17.
Anthonypillai C, Gibbs JE, Thomas SA. The distribution of the anti-HIV drug, tenofovir (PMPA), into the brain, CSF and choroid plexuses. Cerebrospinal Fluid Res. 2006;3:1.
Tashima KT, Caliendo AM, Ahmad M, et al. Cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) suppression and efavirenz drug concentrations in HIV-1-infected patients receiving combination therapy. J Infect Dis. 1999;180:862–4.
Best BM, Koopmans PP, Letendre SL, et al. Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J Antimicrob Chemother. 2010;66:354–7.
van Praag RM, van Weert EC, van Heeswijk RP, et al. Stable concentrations of zidovudine, stavudine, lamivudine, abacavir, and nevirapine in serum and cerebrospinal fluid during 2 years of therapy. Antimicrob Agents Chemother. 2002;46:896–9.
Veldkamp AI, Weverling GJ, Lange JM, et al. High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individuals. AIDS. 2001;15:1089–95.
Mora-Peris B, Watson V, Vera JH, et al. Rilpivirine exposure in plasma and sanctuary site compartments after switching from nevirapine-containing combined antiretroviral therapy. J Antimicrob Chemother. 2014;69(6):1642-7.
Kravcik S, Gallicano K, Roth V, et al. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and saquinavir. J Acquir Immune Defic Syndr. 1999;21:371–5.
Sadler BM, Chittick GE, Polk RE, et al. Metabolic disposition and pharmacokinetics of [14C]-amprenavir, a human immunodeficiency virus type 1 (HIV-1) protease inhibitor, administered as a single oral dose to healthy male subjects. J Clin Pharmacol. 2001;41:386–96.
Murphy R, Currier J, Gerber J, et al. Antiviral activity and pharmacokinetics of amprenavir with or without zidovudine/3TC in the cerebrospinal fluid of HIV-infected adults [abstract]. 7th Conference on Retroviruses and Opportunistic Infections (CROI); 30 Jan–2 Feb 2000; San Francisco.
Saumoy M, Tiraboschi J, Gutierrez M, et al. Viral response in stable patients switching to fosamprenavir/ritonavir monotherapy (the FONT study). HIV Med. 2011;12:438–41.
Yilmaz A, Izadkhashti A, Price RW, et al. Darunavir concentrations in cerebrospinal fluid and blood in HIV-1-infected individuals. AIDS Res Hum Retroviruses. 2009;25:457–61.
Capparelli EV, Holland D, Okamoto C, et al. Lopinavir concentrations in cerebrospinal fluid exceed the 50 % inhibitory concentration for HIV. AIDS. 2005;19:949–52.
DiCenzo R, DiFrancesco R, Cruttenden K, et al. Lopinavir cerebrospinal fluid steady-state trough concentrations in HIV-infected adults. Ann Pharmacother. 2009;43:1972–7.
Lafeuillade A, Solas C, Halfon P, et al. Differences in the detection of three HIV-1 protease inhibitors in non-blood compartments: clinical correlations. HIV Clin Trials. 2002;3:27–35.
Letendre SL, van den Brande G, Hermes A, et al. Lopinavir with ritonavir reduces the HIV RNA level in cerebrospinal fluid. Clin Infect Dis. 2007;45:1511–7.
Yilmaz A, Fuchs D, Hagberg L, et al. Cerebrospinal fluid HIV-1 RNA, intrathecal immunoactivation, and drug concentrations after treatment with a combination of saquinavir, nelfinavir, and two nucleoside analogues: the M61022 study. BMC Infect Dis. 2006;6:63.
Moyle GJ, Sadler M, Buss N. Plasma and cerebrospinal fluid saquinavir concentrations in patients receiving combination antiretroviral therapy. Clin Infect Dis. 1999;28:403–4.
Gisolf EH, Enting RH, Jurriaans S, et al. Cerebrospinal fluid HIV-1 RNA during treatment with ritonavir/saquinavir or ritonavir/saquinavir/stavudine. AIDS. 2000;14:1583–9.
Kim RB, Fromm MF, Wandel C, et al. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–94.
Yilmaz A, Price RW, Gisslén M. Antiretroviral drug treatment of CNS HIV-1 infection. J Antimicrob Chemother. 2012;67(2):299–311.
Price RW, Parham R, Kroll JL, et al. Enfuvirtide cerebrospinal fluid (CSF) pharmacokinetics and potential use in defining CSF HIV-1 origin. Antivir Ther. 2008;13:369–74.
van Lelyveld SF, Nijhuis M, Baatz F, et al. Therapy failure following selection of enfuvirtide-resistant HIV-1 in cerebrospinal fluid. Clin Infect Dis. 2010;50:387–90.
Zhou XJ, Havlir DV, Richman DD, et al. Plasma population pharmacokinetics and penetration into cerebrospinal fluid of indinavir in combination with zidovudine and lamivudine in HIV-1-infected patients. AIDS. 2000;14(18):2869–76.
Letendre SL, Capparelli EV, Ellis RJ, McCutchan JA. Indinavir population pharmacokinetics in plasma and cerebrospinal fluid. Antimicrob Agents Chemother. 2000;44(8):2173–5.
Letendre S, Mills A, Tashima K, et al. Distribution and antiviral activity in cerebrospinal fluid (CSF) of the integrase inhibitor, dolutegravir (DTG): ING116070 week 16 results [abstract]. 20th Conference on Retroviruses and Opportunistic Infections (CROI); 3–6 Mar 2013; Atlanta.
Croteau D, Letendre S, Best BM, et al. Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1. Antimicrob Agents Chemother. 2010;54(12):5156–60.
Staprans S, Marlowe N, Glidden D, et al. Time course of cerebrospinal fluid responses to antiretroviral therapy: evidence for variable compartmentalization of infection. AIDS. 1999;13:1051–61.
Ellis RJ, Gamst AC, Capparelli E, et al. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54:927–36.
Eggers C, Hertogs K, Sturenburg HJ, et al. Delayed central nervous system virus suppression during highly active antiretroviral therapy is associated with HIV encephalopathy, but not with viral drug resistance or poor central nervous system drug penetration. AIDS. 2003;17:1897–906.
Mellgren A, Antinori A, Cinque P, et al. Cerebrospinal fluid HIV-1 infection usually responds well to antiretroviral treatment. Antivir Ther. 2005;10:701–7.
Gisslen M, Norkrans G, Svennerholm B, et al. HIV-1 RNA detectable with ultrasensitive quantitative polymerase chain reaction in plasma but not in cerebrospinal fluid during combination treatment with zidovudine, lamivudine and indinavir. AIDS. 1998;12:114–6.
Letendre S, McClernon D, Ellis R, et al. Persistent HIV in the central nervous system during treatment is associated with worse ART penetration and cognitive impairment [abstract]. 16th Conference on Retroviruses and Opportunistic Infections (CROI); 8–11 Feb 2009; Montreal.
Yilmaz A, Yiannoutsos CT, Fuchs D, et al. Cerebrospinal fluid neopterin decay characteristics after initiation of antiretroviral therapy. J Neuroinflammation. 2013;10:62.
Masters MC, Ances BM. Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol. 2014;34(1):89–102.
Garvey LJ, Pavese N, Politis M, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS. 2014;28(1):67–72.
Robertson KR, Su Z, Margolis DM, et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74(16):1260–6.
Grund B, Wright EJ, Brew BJ, et al. Improved neurocognitive test performance in both arms of the SMART study: impact of practice effect. J Neurovirol. 2013;19(4):383–92.
Acosta EP, Limoli KL, Trinh L, et al. Novel method to assess antiretroviral target trough concentrations using in vitro susceptibility data. Antimicrob Agents Chemother. 2012;56(11):5938–45.
Calcagno A, Simiele M, Alberione MC, et al. Cerebrospinal fluid inhibitory quotients of antiretroviral drugs in HIV-positive patients. Clin Infect Dis. 2014. In Press.
Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59(6):923–8.
Edén A, Hagberg L, Svennerholm B, et al. Longitudinal follow up of Detectable HIV 1 RNA in cerebrospinal fluid in subjects on suppressive antiretroviral therapy [abstract]. 19th Conference on Retroviruses and Opportunistc Infections (CROI); 5–8 Mar 2012; Seattle.
Wendel KA, McArthur JC. Acute meningoencephalitis in chronic human immunodeficiency virus (HIV) infection: putative central nervous system escape of HIV replication. Clin Infect Dis. 2003;37(8):1107–11.
Bogoch II, Davis BT, Venna N. Reversible dementia in a patient with central nervous system escape of human immunodeficiency virus. J Infect. 2011;63(3):236–9.
Bingham R, Ahmed N, Rangi P, et al. HIV encephalitis despite suppressed viraemia: a case of compartmentalized viral escape. Int J STD AIDS. 2011;22(10):608–9.
Khoury MN, Tan CS, Peaslee M, Koralnik IJ. CSF viral escape in a patient with HIV-associated neurocognitive disorder. J Neurovirol. 2013;19(4):402–5.
Cusini A, Vernazza PL, Yerly S, et al. Higher CNS penetration-effectiveness of long-term combination antiretroviral therapy is associated with better HIV-1 viral suppression in cerebrospinal fluid. J Acquir Immune Defic Syndr. 2013;62(1):28–35.
Perez Valero I, Letendre S, Ellis R, et al. Prevalence and risk factors for HIV CSF viral escape: results from the CHARTER and HNRP cohorts. J Int AIDS Soc. 2012;15(Suppl 4):18189.
Gisslén M, Norkrans G, Svennerholm B, Hagberg L. The effect on human immunodeficiency virus type 1 RNA levels in cerebrospinal fluid after initiation of zidovudine or didanosine. J Infect Dis. 1997;175(2):434–7.
Bunupuradah T, Chetchotisakd P, Jirajariyavej S, et al. Neurocognitive impairment in patients randomized to second-line lopinavir/ritonavir-based antiretroviral therapy vs. lopinavir/ritonavir monotherapy. J Neurovirol. 2012;18(6):479–87.
Santos JR, Muñoz-Moreno JA, Moltó J, et al. Virological efficacy in cerebrospinal fluid and neurocognitive status in patients with long-term monotherapy based on lopinavir/ritonavir: an exploratory study. PLoS One. 2013;8(7):e70201.
Pérez-Valero I, González-Baeza A, Estébanez M, et al. Neurocognitive impairment in patients treated with protease inhibitor monotherapy or triple drug antiretroviral therapy. PLoS One. 2013;8(7):e69493.
Perez-Valero I, Bayon C, Cambron I, Gonzalez A, Arribas JR. Protease inhibitor monotherapy and the CNS: peace of mind? J Antimicrob Chemother. 2011;66(9):1954–62.
Katlama C, Valantin MA, Algarte-Genin M, et al. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS. 2010;24:2365–74.
Vernazza P, Daneel S, Schiffer V, et al. The role of compartment penetration in PI-monotherapy: the Atazanavir-Ritonavir Monomaintenance (ATARITMO) Trial. AIDS. 2007;21(10):1309–15.
Du Pasquier RA, Jilek S, Kalubi M, et al. Marked increase of the astrocytic marker S100B in the cerebrospinal fluid of HIV-infected patients on LPV/r-monotherapy. AIDS. 2013;27(2):203–10.
Spudich SS, Nilsson AC, Lollo ND, et al. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis. 2005;5:98.
Yilmaz A, Verhofstede C, D’Avolio A, et al. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;55(5):590–6.
Dahl V, Lee E, Peterson J, et al. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis. 2011;204(12):1936–45.
Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70.
Hammond ER, Crum RM, Treisman GJ, et al. The cerebrospinal fluid HIV risk score for assessing central nervous system activity in persons with HIV. Am J Epidemiol. 2014;180(3):297–307.
Cysique LA, Vaida F, Letendre S, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73(5):342–8.
Tozzi V, Balestra P, Salvatori MF, et al. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr. 2009;52(1):56–63.
Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23(11):1359–66.
Winston A, Duncombe C, Li PC, et al. Does choice of combination antiretroviral therapy (cART) alter changes in cerebral function testing after 48 weeks in treatment-naive, HIV-1-infected individuals commencing cART? A randomized, controlled study. Clin Infect Dis. 2010;50(6):920–9.
Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25(3):357–65.
Arendt G, Orhan E, Nolting T. Retrospective analysis of the HAART CNS penetration effectiveness (CPE) index on neuropsychological performance of a big neuro-AIDS cohort [abstract]. 18th Conference on Retroviruses and Opportunistic Infections (CROI); 27 Feb–2 Mar 2011; Boston.
Garvey L, Surendrakumar V, Winston A. Low rates of neurocognitive impairment are observed in neuro-asymptomatic HIV-infected subjects on effective antiretroviral therapy. HIV Clin Trials. 2011;12(6):333–8.
Rourke SB, Carvalhal A, Zypurski A, et al. CNS penetration effectiveness of cART and neuropsychological outcomes: cross-sectional results from the OHTN Cohort Study [abstract]. 19th Conference on Retroviruses and Opportunistic Infections; 5–8 Mar 2012; Seattle.
Robertson K, Jiang H, Kumwenda J, et al. Improved neuropsychological and neurological functioning across three antiretroviral regimens in diverse resource-limited settings: AIDS Clinical Trials Group study a5199, the International Neurological Study. Clin Infect Dis. 2012;55(6):868–76.
Ciccarelli N, Fabbiani M, Colafigli M, et al. Revised central nervous system neuropenetration-effectiveness score is associated with cognitive disorders in HIV-infected patients with controlled plasma viraemia. Antivir Ther. 2013;18(2):153–60.
Kahouadji Y, Dumurgier J, Sellier P, et al. Cognitive function after several years of antiretroviral therapy with stable central nervous system penetration score. HIV Med. 2013;14(5):311–5.
Ellis RJ, Letendre S, Vaida F, et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis. 2014;58(7):1015–22.
Vassallo M, Durant J, Biscay V, et al. Can high central nervous system penetrating antiretroviral regimens protect against the onset of HIV-associated neurocognitive disorders? AIDS. 2014;28(4):493–501.
Antinori A, Lorenzini P, Giancola ML, et al. Antiretroviral CNS penetration-effectiveness (CPE) 2010 ranking predicts CSF viral suppression only in patients with undetectable HIV-1 RNA in plasma [abstract]. 18th Conference on Retroviruses and Opportunistic Infections (CROI); 27 Feb–2 Mar 2011; Boston.
Giancola ML, Lorenzini P, Cingolani A, et al. virological response in cerebrospinal fluid to antiretroviral therapy in a large Italian cohort of HIV-infected patients with neurological disorders. AIDS Res Treat. 2012;2012:708456.
Rawson T, Muir D, Mackie NE, et al. Factors associated with cerebrospinal fluid HIV RNA in HIV infected subjects undergoing lumbar puncture examination in a clinical setting. J Infect. 2012;65(3):239–45.
Pinnetti C, Lorenzini P, Forbici F, et al. CSF viral escape in patients without neurological disorders: prevalence and associated factors [abstract]. 20th Conference on Retroviruses and Opportunistic Infections (CROI); 3–6 Mar 2014; Boston.
Casado JL, Marín A, Moreno A, Iglesias V, Perez-Elías MJ, Moreno S, Corral I. Central nervous system antiretroviral penetration and cognitive functioning in largely pretreated HIV-infected patients. J Neurovirol. 2014;20(1):54–61.
Fabbiani M, Grima P, Milanini B, et al. Central nervous system penetration effectiveness score better correlates with cognitive performance of HIV+ patients after accounting for drug susceptibility of plasma virus [abstract]. 19th Conference on Retroviruses and Opportunistic Infections (CROI); 5–8 Mar 2012; Seattle.
Antinori A, Marcotullio S, Ammassari A, et al. Italian guidelines for the use of antiretroviral agents and the diagnostic-clinical management of HIV-1 infected persons. Update 2011. New Microbiol. 2012;35(2):113–59.
Cysique LA, Waters EK, Brew BJ. Central nervous system antiretroviral efficacy in HIV infection: a qualitative and quantitative review and implications for future research. BMC Neurol. 2011;11:148.
Aquaro S, Svicher V, Schols D, et al. Mechanisms underlying activity of antiretroviral drugs in HIV-1-infected macrophages: new therapeutic strategies. J Leukoc Biol. 2006;80(5):1103–10.
Shikuma CM, Nakamoto B, Shiramizu B, et al. Antiretroviral monocyte efficacy score linked to cognitive impairment in HIV. Antivir Ther. 2012;17(7):1233–42.
Gray LR, Tachedjian G, Ellett AM, et al. The NRTIs lamivudine, stavudine and zidovudine have reduced HIV-1 inhibitory activity in astrocytes. PLoS One. 2013;8(4):e62196.
Kallianpur KJ, Shikuma C, Kirk GR, et al. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology. 2013;80(19):1792–9.
Valcour VG, Ananworanich J, Agsalda M, et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naïve patients. PLoS One. 2013;8(7):e70164.
Rusconi S, Vitiello P, Adorni F, et al. Maraviroc as intensification strategy in HIV-1 positive patients with deficient immunological response: an Italian randomized clinical trial. PLoS One. 2013;8(11):e80157.
Soulié C, Tubiana R, Simon A, et al. Presence of HIV-1 R5 viruses in cerebrospinal fluid even in patients harboring R5X4/X4 viruses in plasma. J Acquir Immune Defic Syndr. 2009;51(1):60–4.
Garvey L, Nelson M, Latch N, et al. CNS effects of a CCR5 inhibitor in HIV-infected subjects: a pharmacokinetic and cerebral metabolite study. J Antimicrob Chemother. 2012;67(1):206–12.
Vera JH, Garvey LJ, Allsop JM, et al. Alterations in cerebrospinal fluid chemokines are associated with maraviroc exposure and in vivo metabolites measurable by magnetic resonance spectroscopy. HIV Clin Trials. 2012;13(4):222–7.
Cui L, Locatelli L, Xie MY, Sommadossi JP. Effect of nucleoside analogs on neurite regeneration and mitochondrial DNA synthesis in PC-12 cells. J Pharmacol Exp Ther. 1997;280:1228–34.
Werth JL, Zhou B, Nutter LM, Thayer SA. 2′,3′-Dideoxycytidine alters calcium buffering in cultured dorsal root ganglion neurons. Mol Pharmacol. 1994;45:1119–24.
Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18(5):388–99.
Akay C, Cooper M, Odeleye A, et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014;20(1):39–53.
Friis-Møller N, Thiébaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491–501.
Cruse B, Cysique LA, Markus R, Brew BJ. Cerebrovascular disease in HIV-infected individuals in the era of highly active antiretroviral therapy. J Neurovirol. 2012;18:264–76.
Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007;68:1257–61.
Soontornniyomkij V, Umlauf A, Chung SA, et al.HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014;28(9):1297-306.
Green DA, Masliah E, Vinters HV, et al. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19(4):407–11.
Giunta B, Ehrhart J, Obregon DF, et al. Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Mol Brain. 2011;4(1):23.
Ciccarelli N, Fabbiani M, Di Giambenedetto S, et al. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76(16):1403–9.
Conflict of interest
No funding has been received for the preparation of this review.
A. Calcagno has received travel grants or speaker’s honoraria from Abbott, Bristol-Myers Squibb (BMS), Merck Sharp & Dohme (MSD) and Janssen-Cilag. S. Bonora has received grants, travel grants and consultancy fees from Abbott, Boehringer-Inghelheim, BMS, Gilead-Sciences, GlaxoSmithKline (GSK), MSD, Pfizer and Janssen-Cilag. G. Di Perri has received grants, travel frants and consultancy fees from Abbott, Boehringer-Inghelheim, BMS, Gilead-Sciences, GSK, MSD, Pfizer, Roche and Tibotec (Johnson & Johnson).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calcagno, A., Di Perri, G. & Bonora, S. Pharmacokinetics and Pharmacodynamics of Antiretrovirals in the Central Nervous System. Clin Pharmacokinet 53, 891–906 (2014). https://doi.org/10.1007/s40262-014-0171-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0171-0