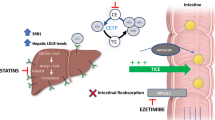
Abstract
In almost 30 years since the introduction of HMG-CoA reductase inhibitors (statins), no other class of lipid modulators has entered the market. Elevation of high-density lipoprotein-cholesterol (HDL-C) via inhibiting cholesteryl ester transfer protein (CETP) is an attractive strategy for reducing the risk of cardiovascular events in high-risk patients. Transfer of triglyceride and cholesteryl ester (CE) between lipoproteins is mediated by CETP; thus inhibition of this pathway can increase the concentration of HDL-C. Torcetrapib was the first CETP inhibitor evaluated in phase III clinical trials. Because of off-target effects, torcetrapib raised blood pressure and increased the concentration of serum aldosterone, leading to higher cardiovascular events and mortality. Torcetrapib showed positive effects on cardiovascular risk especially in patients with a greater increase in HDL-C and apolipoprotein A-1 (apoA-1) levels. The phase III clinical trial of dalcetrapib, the second CETP inhibitor that has entered clinical development, was terminated because of ineffectiveness. Dalcetrapib is a CETP modulator that elevated HDL-C levels but did not reduce the concentration of low-density lipoprotein cholesterol (LDL-C). Both heterotypic and homotypic CE transfer between lipoproteins are mediated by some CETP inhibitors, including torcetrapib, anacetrapib, and evacetrapib, while dalcetrapib only affects the heterotypic CE transfer. Dalcetrapib has a chemical structure that is distinct from other CETP inhibitors, with a smaller molecular weight and a lack of trifluoride moieties. Moreover, dalcetrapib is a pro-drug that must be hydrolyzed to a pharmacologically active thiol form. Two other CETP inhibitors, anacetrapib and evacetrapib, are currently undergoing evaluation in phase III clinical trials. Both molecules have shown beneficial effects by increasing HDL-C and decreasing LDL-C concentration. The success of anacetrapib and evacetrapib remains to be confirmed upon the completion of phase III clinical trials in 2017 and 2015, respectively. Generally, the concentration of HDL-C has been considered a biomarker for the activity of CETP inhibitors. However, it is not clear whether a fundamental relationship exists between HDL-C levels and the risk of coronary artery diseases. The most crucial role for HDL is cholesterol efflux capacity in which HDL can reverse transport cholesterol from foam cells in atherosclerotic plaques. In view of the heterogeneity in HDL particle size, charge, and composition, the mere concentration of HDL-C may not be a good surrogate marker for HDL functionality. Recent clinical studies have reported that increased HDL functionality inversely correlates with the development of atherosclerotic plaque. Future development of CETP inhibitors may therefore benefit from the use of biomarkers of HDL functionality.


Similar content being viewed by others
Reference
Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham study. Am J Med. 1977;62(5):707–14.
Castelli WP, Garrison RJ, Wilson PW, et al. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256(20):2835–8.
Corti MC, Guralnik JM, Salive ME, et al. HDL cholesterol predicts coronary heart disease mortality in older persons. JAMA. 1995;274(7):539–44.
Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15.
Kuvin JT, Alsheikh-Ali AA, Karas RH. High-density lipoprotein cholesterol-raising strategies. J Cardiovasc Pharmacol. 2006;47(2):196–204.
Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–35.
Staels B, Dallongeville J, Auwerx J, et al. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088–93.
Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341(6):410–8.
Shepherd J. Fibrates and statins in the treatment of hyperlipidaemia: an appraisal of their efficacy and safety. Eur Heart J. 1995;16(1):5–13.
Bruckert E, Labreuche J, Amarenco P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 2010;210(2):353–61.
Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298(7):786–98.
Lagrost L, Gambert P, Dangremont V, et al. Role of cholesteryl ester transfer protein (CETP) in the HDL conversion process as evidenced by using anti-CETP monoclonal antibodies. J Lipid Res. 1990;31(9):1569–75.
Okamoto H, Yonemori F, Wakitani K, et al. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 2000;406(6792):203–7.
Clark RW, Ruggeri RB, Cunningham D, et al. Description of the torcetrapib series of cholesteryl ester transfer protein inhibitors, including mechanism of action. J Lipid Res. 2006;47(3):537–52.
Schwartz GG, Olsson AG, Ballantyne CM, et al. Rationale and design of the dal-OUTCOMES trial: efficacy and safety of dalcetrapib in patients with recent acute coronary syndrome. Am Heart J. 2009;158(6):896–901, e3.
Krishna R, Anderson MS, Bergman AJ, et al. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet. 2007;370(9603):1907–14.
Cao G, Beyer TP, Zhang Y, et al. Evacetrapib is a novel, potent, and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure. J Lipid Res. 2011;52(12):2169–76.
Weber O, Willmann S, Bischoff H, et al. Prediction of a potentially effective dose in humans for BAY 60–5521, a potent inhibitor of cholesteryl ester transfer protein (CETP) by allometric species scaling and combined pharmacodynamic and physiologically-based pharmacokinetic modelling. Br J Clin Pharmacol. 2012;73(2):219–31.
Sarich TC, Connelly MA, Schranz DB, et al. A Phase 0 study of the inhibition of cholesteryl ester transfer protein activity by JNJ-28545595 in plasma from normolipidemic and dyslipidemic humans. Int J Clin Pharmacol Ther. 2012;50(8):584–94.
Tall AR, Yvan-Charvet L, Wang N. The failure of torcetrapib: was it the molecule or the mechanism? Arterioscler Thromb Vasc Biol. 2007;27(2):257–60.
Sweetlove M. Phase III trial of dalcetrapib: discontinued due to lack of efficacy. Pharm Med. 2012;26(4):253–6.
Shinkai H. Cholesteryl ester transfer-protein modulator and inhibitors and their potential for the treatment of cardiovascular diseases. Vasc Health Risk Manag. 2012;8:323–31.
Masson D, Jiang XC, Lagrost L, et al. The role of plasma lipid transfer proteins in lipoprotein metabolism and atherogenesis. J Lipid Res. 2009;50(Suppl):S201–6.
Barter PJ, Rye KA. Cholesteryl ester transfer protein (CETP) inhibition as a strategy to reduce cardiovascular risk. J Lipid Res. 2012;53(9):1755–66.
Boekholdt SM, Kuivenhoven JA, Hovingh GK, et al. CETP gene variation: relation to lipid parameters and cardiovascular risk. Curr Opin Lipidol. 2004;15(4):393–8.
Thompson A, Di Angelantonio E, Sarwar N, et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 2008;299(23):2777–88.
Brown ML, Inazu A, Hesler CB, et al. Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteins. Nature. 1989;342(6248):448–51.
Inazu A, Brown ML, Hesler CB, et al. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med. 1990;323(18):1234–8.
Boekholdt SM, Thompson JF. Natural genetic variation as a tool in understanding the role of CETP in lipid levels and disease. J Lipid Res. 2003;44(6):1080–93.
Ridker PM, Pare G, Parker AN, et al. Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: Genomewide analysis among 18245 initially healthy women from the Women’s Genome Health Study. Circ Cardiovasc Genet. 2009;2(1):26–33.
Wolk R, Chen D, Clark RW, et al. Pharmacokinetic, pharmacodynamic, and safety profile of a new cholesteryl ester transfer protein inhibitor in healthy human subjects. Clin Pharmacol Ther. 2009;86(4):430–7.
Dalvie D, Chen W, Zhang C, et al. Pharmacokinetics, metabolism, and excretion of torcetrapib, a cholesteryl ester transfer protein inhibitor, in humans. Drug Metab Dispos. 2008;36(11):2185–98.
Prakash C, Chen W, Rossulek M, et al. Metabolism, pharmacokinetics, and excretion of a cholesteryl ester transfer protein inhibitor, torcetrapib, in rats, monkeys, and mice: characterization of unusual and novel metabolites by high-resolution liquid chromatography-tandem mass spectrometry and 1H nuclear magnetic resonance. Drug Metab Dispos. 2008;36(10):2064–79.
Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356(13):1304–16.
Kastelein JJ, van Leuven SI, Burgess L, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356(16):1620–30.
Bots ML, Visseren FL, Evans GW, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 2007;370(9582):153–60.
Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–22.
Rader DJ. Illuminating HDL—is it still a viable therapeutic target? N Engl J Med. 2007;357(21):2180–3.
Hewing B, Fisher EA. Rationale for cholesteryl ester transfer protein inhibition. Curr Opin Lipidol. 2012;23(4):372–6.
Bentley D, Young AM, Rowell L, et al. Evidence of a drug–drug interaction linked to inhibition of ester hydrolysis by orlistat. J Cardiovasc Pharmacol. 2012;60(4):390–6.
Vergeer M, Stroes ES. The pharmacology and off-target effects of some cholesterol ester transfer protein inhibitors. Am J Cardiol. 2009;104(10 Suppl):32E–8E.
Derks M, Anzures-Cabrera J, Turnbull L, et al. Safety, tolerability and pharmacokinetics of dalcetrapib following single and multiple ascending doses in healthy subjects: a randomized, double-blind, placebo-controlled, phase I study. Clin Drug Investig. 2011;31(5):325–35.
Niesor EJ, Magg C, Ogawa N, et al. Modulating cholesteryl ester transfer protein activity maintains efficient pre-beta-HDL formation and increases reverse cholesterol transport. J Lipid Res. 2010;51(12):3443–54.
Kuhlmann O, Heinig K. Dalcetrapib pharmacokinetics and metabolism in the cynomolgus monkey. Xenobiotica. 2011;41(5):430–6.
Derks M, Kawamura H, Abt M, et al. Effects of food intake on the pharmacokinetic properties of dalcetrapib: findings from three phase I, single-dose crossover studies in healthy volunteers. Clin Ther. 2011;33(6):754–65.
Xiao D, Shi D, Yang D, et al. Carboxylesterase-2 is a highly sensitive target of the antiobesity agent orlistat with profound implications in the activation of anticancer prodrugs. Biochem Pharmacol. 2013;85(3):439–47.
Derks M, Abt M, Phelan M, et al. Coadministration of dalcetrapib with pravastatin, rosuvastatin, or simvastatin: no clinically relevant drug–drug interactions. J Clin Pharmacol. 2010;50(10):1188–201.
Derks M, Abt M, Parr G, et al. No clinically relevant drug–drug interactions when dalcetrapib is co-administered with atorvastatin. Expert Opin Investig Drugs. 2010;19(10):1135–45.
Derks M, Abt M, Phelan M. Lack of clinically relevant drug–drug interactions when dalcetrapib is co-administered with ezetimibe. Br J Clin Pharmacol. 2010;70(6):825–33.
Young A, Anzures-Cabrera J, Derks M. No clinically relevant drug-drug interactions when dalcetrapib is co-administered with a monophasic oral contraceptive (Microgynon(R) 30). Int J Clin Pharmacol Ther. 2012;50(4):248–56.
Baldo PA, Anzures-Cabrera J, Bentley D. In vivo evaluation of drug-drug interactions linked to UGT inhibition: the effect of probenecid on dalcetrapib pharmacokinetics. Int J Clin Pharmacol Ther. 2013;51(3):215–8.
Luscher TF, Taddei S, Kaski JC, et al. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur Heart J. 2012;33(7):857–65.
Fayad ZA, Mani V, Woodward M, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378(9802):1547–59.
Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–99.
Krishna R, Garg A, Panebianco D, et al. Single-dose pharmacokinetics and pharmacodynamics of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Br J Clin Pharmacol. 2009;68(4):535–45.
Krishna R, Bergman AJ, Jin B, et al. Multiple-dose pharmacodynamics and pharmacokinetics of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Clin Pharmacol Ther. 2008;84(6):679–83.
Tan EY, Hartmann G, Chen Q, et al. Pharmacokinetics, metabolism, and excretion of anacetrapib, a novel inhibitor of the cholesteryl ester transfer protein, in rats and rhesus monkeys. Drug Metab Dispos. 2010;38(3):459–73.
Kumar S, Tan EY, Hartmann G, et al. Metabolism and excretion of anacetrapib, a novel inhibitor of the cholesteryl ester transfer protein, in humans. Drug Metab Dispos. 2010;38(3):474–83.
Krishna R, Garg A, Jin B, et al. Assessment of a pharmacokinetic and pharmacodynamic interaction between simvastatin and anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy subjects. Br J Clin Pharmacol. 2009;67(5):520–6.
Krishna R, Stypinski D, Ali M, et al. Lack of an effect of anacetrapib on the pharmacokinetics of digoxin in healthy subjects. Biopharm Drug Dispos. 2011;32(9):525–9.
Krishna R, Stypinski D, Ali M, et al. Lack of a meaningful effect of anacetrapib on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2012;74(1):116–24.
Krishna R, Bergman AJ, Jin B, et al. Assessment of the CYP3A-mediated drug interaction potential of anacetrapib, a potent cholesteryl ester transfer protein (CETP) inhibitor, in healthy volunteers. J Clin Pharmacol. 2009;49(1):80–7.
Yvan-Charvet L, Kling J, Pagler T, et al. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler Thromb Vasc Biol. 2010;30(7):1430–8.
Cannon CP, Shah S, Dansky HM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363(25):2406–15.
Krauss RM, Wojnooski K, Orr J, et al. Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib. J Lipid Res. 2012;53(3):540–7.
Lamarche B, Tchernof A, Moorjani S, et al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997;95(1):69–75.
Fernandez MC, Escribano A, Mateo AI, et al. Design, synthesis and structure-activity-relationship of 1,5-tetrahydronaphthyridines as CETP inhibitors. Bioorg Med Chem Lett. 2012;22(9):3056–62.
Nicholls SJ, Brewer HB, Kastelein JJ, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA. 2011;306(19):2099–109.
Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–80.
Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis. 1988;8(6):737–41.
Franceschini G, Sirtori CR, Capurso A 2nd, et al. A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J Clin Invest. 1980;66(5):892–900.
Calabresi L, Baldassarre D, Castelnuovo S, et al. Functional lecithin: cholesterol acyltransferase is not required for efficient atheroprotection in humans. Circulation. 2009;120(7):628–35.
Frikke-Schmidt R, Nordestgaard BG, Stene MC, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA. 2008;299(21):2524–32.
Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263(3):256–73.
Linsel-Nitschke P, Jansen H, Aherrarhou Z, et al. Macrophage cholesterol efflux correlates with lipoprotein subclass distribution and risk of obstructive coronary artery disease in patients undergoing coronary angiography. Lipids Health Dis. 2009;8:14.
Mikkola TS, Anthony MS, Clarkson TB, et al. Serum cholesterol efflux potential is an independent predictor of coronary artery atherosclerosis. Atherosclerosis. 2003;170(1):31–8.
Low H, Hoang A, Sviridov D. Cholesterol efflux assay. J Vis Exp. 2012;61:e3810.
Daniil G, Phedonos AA, Holleboom AG, et al. Characterization of antioxidant/anti-inflammatory properties and apoA-I-containing subpopulations of HDL from family subjects with monogenic low HDL disorders. Clin Chim Acta. 2011;412(13–14):1213–20.
Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299(11):1265–76.
Barter PJ, Nicholls S, Rye KA, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95(8):764–72.
Krukemyer JJ, Talbert RL. Lovastatin: a new cholesterol-lowering agent. Pharmacotherapy. 1987;7(6):198–210.
Zhao XQ, Morse JS, Dowdy AA, et al. Safety and tolerability of simvastatin plus niacin in patients with coronary artery disease and low high-density lipoprotein cholesterol. The HDL Atherosclerosis Treatment Study. Am J Cardiol. 2004;93:307–12.
Acknowledgments
Support from Grant # R15 GM101599 from the National Institutes of Health is gratefully acknowledged. The authors (Mohammadpour AH and Akhlaghi F) declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammadpour, A.H., Akhlaghi, F. Future of Cholesteryl Ester Transfer Protein (CETP) Inhibitors: A Pharmacological Perspective. Clin Pharmacokinet 52, 615–626 (2013). https://doi.org/10.1007/s40262-013-0071-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-013-0071-8