Abstract
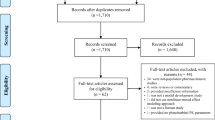
Three decades after its introduction, pharmacokinetic population approaches have become a reference method for drug modelling, particularly in paediatrics. The main practical limitation in this specific population is the collected blood volume. Pharmacokinetic population approaches using sparse sampling may resolve this issue. The pharmacokinetics of many drugs have been studied during the last 25 years using such methods. This review summarizes all of the published studies concerning population pharmacokinetic approaches in paediatric subjects from neonate to 2 years old. A literature search was conducted using the PubMed database, from 1985 to December 2010, using the following terms: pharmacokinetic(s), population, paediatric/pediatric and neonate(s). Articles were excluded if they were not pertinent according to our criteria. References of all relevant articles were also evaluated. Ninety-eight studies were included in this review. The following information was extracted from the articles: drug name, therapeutic class, population size, age of patients, number of samples per patient, covariates used for clearance and volume of distribution estimates, software used for modelling and validation methods. An increasing rate of publications over the years was observed; 44 different drugs were studied using a pharmacokinetic population approach. Antibacterials were the most studied class of drugs, including a large number of studies devoted to vancomycin and gentamicin. It must be underlined that few studies have been performed on anticonvulsant drugs and anaesthetics used in clinical daily practice conditions.




Similar content being viewed by others
References
Steinbrook R. Testing medications in children. N Engl J Med. 2002;347:1462–70.
Rosato J. The ethics of clinical trials: a child’s view. J Law Med Ethics. 2000;28:362–78.
Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ. 2000;320:79–82.
Treluyer JM, Berger JF, Leclerc F, et al. Use of off-label and unlicensed drugs in neonatal and paediatric intensive care in France [abstract no. 46A]. Pediatric Academic Societies Annual Meeting; 1–4 May 1999; San Francisco.
Chalumeau M, Treluyer JM, Salenave B, et al. Off label and unlicensed drug use among office-based paediatricians. Arch Dis Child. 2000;82:502–5.
Anderson GD, Lynn AM. Optimizing pediatric dosing: a developmental pharmacologic approach. Pharmacotherapy. 2009;29(6):680–90.
Anderson BJ. My child is unique; the pharmacokinetics are universal. Paediatr Anaesth. 2012;22(6):530–8.
Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, et al. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet. 2007;46(3):221–34.
Tod M, Jullien V, Pons G. Facilitation of drug evaluation in children by population methods and modelling. Clin Pharmacokinet. 2008;47(4):231–43.
Grasela TH Jr, Donn SM. Neonatal population pharmacokinetics of phenobarbital derived from routine clinical data. Dev Pharmacol Ther. 1985;8(6):374–83.
Schaible DH, Rocci ML Jr, Alpert GA, Campos JM, Paul MH, Polin RA, et al. Vancomycin pharmacokinetics in infants: relationships to indices of maturation. Pediatr Infect Dis. 1986;5(3):304–8.
Thomson AH, Way S, Bryson SM, McGovern EM, Kelman AW, Whiting B. Population pharmacokinetics of gentamicin in neonates. Dev Pharmacol Ther. 1988;11(3):173–9.
Moore ES, Faix RG, Banagale RC, Grasela TH. The population pharmacokinetics of theophylline in neonates and young infants. J Pharmacokinet Biopharm. 1989;17(1):47–66.
Wiest DB, Pinson JB, Gal PS, Brundage RC, Schall S, Ransom JL, et al. Population pharmacokinetics of intravenous indomethacin in neonates with symptomatic patent ductus arteriosus. Clin Pharmacol Ther. 1991;49(5):550–7.
Karlsson MO, Thomson AH, McGovern EM, Chow P, Evans TJ, Kelman AW. Population pharmacokinetics of rectal theophylline in neonates. Ther Drug Monit. 1991;13(3):195–200.
Fattinger K, Vozeh S, Olafsson A, Vlcek J, Wenk M, Follath F. Netilmicin in the neonate: population pharmacokinetic analysis and dosing recommendations. Clin Pharmacol Ther. 1991;50(1):55–65.
Izquierdo M, Lanao JM, Cervero L, Jimenez NV, Domínguez-Gil A. Population pharmacokinetics of gentamicin in premature infants. Ther Drug Monit. 1992;14(3):177–83.
Jensen PD, Edgren BE, Brundage RC. Population pharmacokinetics of gentamicin in neonates using a nonlinear, mixed-effects model. Pharmacotherapy. 1992;12(3):178–82.
Karna P, Lee C, Kumar A, Dyke J, Gooch WM 3rd. Population pharmacokinetics of ceftizoxime in premature newborns. Dev Pharmacol Ther. 1993;20(3–4):135–43.
Weber W, Kewitz G, Rost KL, Looby M, Nitz M, Harnisch L. Population kinetics of gentamicin in neonates. Eur J Clin Pharmacol. 1993;44(Suppl 1):S23–5.
Asbury WH, Darsey EH, Rose WB, Murphy JE, Buffington DE, Capers CC. Vancomycin pharmacokinetics in neonates and infants: a retrospective evaluation. Ann Pharmacother. 1993;27(4):490–6.
Burtin P, Jacqz-Aigrain E, Girard P, Lenclen R, Magny JF, Betremieux P, et al. Population pharmacokinetics of midazolam in neonates. Clin Pharmacol Ther. 1994;56(6 Pt 1):615–25.
Seay RE, Brundage RC, Jensen PD, Schilling CG, Edgren BE. Population pharmacokinetics of vancomycin in neonates. Clin Pharmacol Ther. 1994;56(2):169–75.
Lee TC, Charles BG, Steer PA, Flenady VJ, Grant TC. Theophylline population pharmacokinetics from routine monitoring data in very premature infants with apnoea. Br J Clin Pharmacol. 1996;41(3):191–200.
Zhou XJ, Gruber W, Demmler G, Jacobs R, Reuman P, Adler S, et al. Population pharmacokinetics of ganciclovir in newborns with congenital cytomegalovirus infections: NIAID Collaborative Antiviral Study Group. Antimicrob Agents Chemother. 1996;40(9):2202–5.
Thomson AH, Kerr S, Wright S. Population pharmacokinetics of caffeine in neonates and young infants. Ther Drug Monit. 1996;18(3):245–53.
Harte GJ, Gray PH, Lee TC, Steer PA, Charles BG. Haemodynamic responses and population pharmacokinetics of midazolam following administration to ventilated, preterm neonates. J Paediatr Child Health. 1997;33(4):335–8.
Charles BG, Preechagoon Y, Lee TC, Steer PA, Flenady VJ, Debuse N. Population pharmacokinetics of intravenous amoxicillin in very low birth weight infants. J Pharm Sci. 1997;86(11):1288–92.
de Hoog M, Schoemaker RC, Mouton JW, van den Anker JN. Tobramycin population pharmacokinetics in neonates. Clin Pharmacol Ther. 1997;62(4):392–9.
Falcão AC, de Fernández Gatta MM, Delgado Iribarnegaray MF, Santos Buelga D, García MJ, Dominguez-Gil A, et al. Population pharmacokinetics of caffeine in premature neonates. Eur J Clin Pharmacol. 1997;52(3):211–7.
Lee TC, Charles B, Steer P, Flenady V, Shearman A. Population pharmacokinetics of intravenous caffeine in neonates with apnea of prematurity. Clin Pharmacol Ther. 1997;61(6):628–40.
Botha JH, du Preez MJ, Miller R, Adhikari M. Determination of population pharmacokinetic parameters for amikacin in neonates using mixed-effect models. Eur J Clin Pharmacol. 1998;53(5):337–41.
Yasuhara M, Iga T, Zenda H, Okumura K, Oguma T, Yano Y, Hori R. Population pharmacokinetics of vancomycin in Japanese pediatric patients. Ther Drug Monit. 1998;20(6):612–8.
Silva R, Reis E, Bispo MA, Almeida AM, Costa IM, Falcão F, et al. The kinetic profile of vancomycin in neonates. J Pharm Pharmacol. 1998;50(11):1255–60.
Lee TC, Charles BG, Harte GJ, Gray PH, Steer PA, Flenady VJ. Population pharmacokinetic modeling in very premature infants receiving midazolam during mechanical ventilation: midazolam neonatal pharmacokinetics. Anesthesiology. 1999;90(2):451–7.
du Preez MJ, Botha JH, McFadyen ML, Holford NH. The pharmacokinetics of theophylline in premature neonates during the first few days after birth. Ther Drug Monit. 1999;21(6):598–603.
Vervelde ML, Rademaker CM, Krediet TG, Fleer A, van Asten P, van Dijk A. Population pharmacokinetics of gentamicin in preterm neonates: evaluation of a once-daily dosage regimen. Ther Drug Monit. 1999;21(5):514–9.
Grimsley C, Thomson AH. Pharmacokinetics and dose requirements of vancomycin in neonates. Arch Dis Child Fetal Neonatal Ed. 1999;81(3):F221–7.
Preechagoon Y, Charles B, Piotrovskij V, Donovan T, Van Peer A. Population pharmacokinetics of enterally administered cisapride in young infants with gastro-oesophageal reflux disease. Br J Clin Pharmacol. 1999;48(5):688–93.
Mirochnick M, Capparelli E, Connor J. Pharmacokinetics of zidovudine in infants: a population analysis across studies. Clin Pharmacol Ther. 1999;66(1):16–24.
Wang J, Liang WQ, Wu JJ, Pan CM. Population pharmacokinetic analysis of amikacin and validation on neonates using Monte Carlo method. Acta Pharmacol Sin. 2000;21(10):954–60.
de Hoog M, Schoemaker RC, Mouton JW, van den Anker JN. Vancomycin population pharmacokinetics in neonates. Clin Pharmacol Ther. 2000;67(4):360–7.
Hansen TG, Ilett KF, Reid C, Lim SI, Hackett LP, Bergesio R. Caudal ropivacaine in infants: population pharmacokinetics and plasma concentrations. Anesthesiology. 2001;94(4):579–84.
Bleyzac N, Varnier V, Labaune JM, Corvaisier S, Maire P, Jelliffe RW, et al. Population pharmacokinetics of amikacin at birth and interindividual variability in renal maturation. Eur J Clin Pharmacol. 2001;57(6–7):499–504.
Kimura T, Kokubun H, Nowatari M, Matsuura N, Sunakawa K, Kubo H. Population pharmacokinetics of panipenem in neonates and retrospective evaluation of dosage. J Antimicrob Chemother. 2001;47(1):51–9.
Falcão AC, Buelga DS, Méndez ME, García MJ, Pardo M. Population kinetics of tobramycin in neonates. Ther Drug Monit. 2001;23(3):202–8.
Capparelli EV, Lane JR, Romanowski GL, McFeely EJ, Murray W, Sousa P, et al. The influences of renal function and maturation on vancomycin elimination in newborns and infants. J Clin Pharmacol. 2001;41(9):927–34.
Tod M, Lokiec F, Bidault R, De Bony F, Petitjean O, Aujard Y. Pharmacokinetics of oral acyclovir in neonates and in infants: a population analysis. Antimicrob Agents Chemother. 2001;45(1):150–7.
Suematsu F, Yukawa E, Yukawa M, Minemoto M, Ohdo S, Higuchi S, et al. Population-based investigation of relative clearance of digoxin in Japanese neonates and infants by multiple-trough screen analysis. Eur J Clin Pharmacol. 2001;57(1):19–24.
Stolk LM, Degraeuwe PL, Nieman FH, de Wolf MC, de Boer A. Population pharmacokinetics and relationship between demographic and clinical variables and pharmacokinetics of gentamicin in neonates. Ther Drug Monit. 2002;24(4):527–31.
de Hoog M, Schoemaker RC, van den Anker JN, Vinks AA. NONMEM and NPEM2 population modeling: a comparison using tobramycin data in neonates. Ther Drug Monit. 2002;24(3):359–65.
Odoul F, Le Guellec C, Henrot A, Saliba E, Levron JC, Saux MC, et al. Population pharmacokinetics of cisapride in neonates. Eur J Clin Pharmacol. 2002;58(8):507–13.
Mulla H, McCormack P, Lawson G, Firmin RK, Upton DR. Pharmacokinetics of midazolam in neonates undergoing extracorporeal membrane oxygenation. Anesthesiology. 2003;99(2):275–82.
Botha JH, du Preez MJ, Adhikari M. Population pharmacokinetics of gentamicin in South African newborns. Eur J Clin Pharmacol. 2003;59(10):755–9.
DiCenzo R, Forrest A, Slish JC, Cole C, Guillet R. A gentamicin pharmacokinetic population model and once-daily dosing algorithm for neonates. Pharmacotherapy. 2003;23(5):585–91.
Gregoire N, Gualano V, Geneteau A, Millerioux L, Brault M, Mignot A, et al. Population pharmacokinetics of ibuprofen enantiomers in very premature neonates. J Clin Pharmacol. 2004;44(10):1114–24.
Smyth JM, Collier PS, Darwish M, Millership JS, Halliday HL, Petersen S, et al. Intravenous indometacin in preterm infants with symptomatic patent ductus arteriosus: a population pharmacokinetic study. Br J Clin Pharmacol. 2004;58(3):249–58.
Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92(2):208–17.
Rapp HJ, Molnár V, Austin S, Krohn S, Gädeke V, Motsch J, et al. Ropivacaine in neonates and infants: a population pharmacokinetic evaluation following single caudal block. Paediatr Anaesth. 2004;14(9):724–32.
Allegaert K, Anderson BJ, Naulaers G, de Hoon J, Verbesselt R, Debeer A, et al. Intravenous paracetamol (propacetamol) pharmacokinetics in term and preterm neonates. Eur J Clin Pharmacol. 2004;60(3):191–7.
Kimura T, Sunakawa K, Matsuura N, Kubo H, Shimada S, Yago K. Population pharmacokinetics of arbekacin, vancomycin, and panipenem in neonates. Antimicrob Agents Chemother. 2004;48(4):1159–67.
Lanao JM, Calvo MV, Mesa JA, Martín-Suárez A, Carbajosa MT, Miguelez F, et al. Pharmacokinetic basis for the use of extended interval dosage regimens of gentamicin in neonates. J Antimicrob Chemother. 2004;54(1):193–8.
Bailey JM, Hoffman TM, Wessel DL, Nelson DP, Atz AM, Chang AC, et al. A population pharmacokinetic analysis of milrinone in pediatric patients after cardiac surgery. J Pharmacokinet Pharmacodyn. 2004;31(1):43–59.
Chalkiadis GA, Anderson BJ, Tay M, Bjorksten A, Kelly JJ. Pharmacokinetics of levobupivacaine after caudal epidural administration in infants less than 3 months of age. Br J Anaesth. 2005;95(4):524–9.
Fukuda T, Yukawa E, Kondo G, Maeda T, Shin-o T, Kondo Y, et al. Population pharmacokinetics of theophylline in very premature Japanese infants with apnoea. J Clin Pharm Ther. 2005;30(6):591–6.
Capparelli E, Hochwald C, Rasmussen M, Parham A, Bradley J, Moya F. Population pharmacokinetics of cefepime in the neonate. Antimicrob Agents Chemother. 2005;49(7):2760–6.
Yukawa E, Suematsu F, Yukawa M, Minemoto M. Population pharmacokinetic investigation of phenobarbital by mixed effect modelling using routine clinical pharmacokinetic data in Japanese neonates and infants. J Clin Pharm Ther. 2005;30(2):159–63.
Würthwein G, Groll AH, Hempel G, Adler-Shohet FC, Lieberman JM, Walsh TJ. Population pharmacokinetics of amphotericin B lipid complex in neonates. Antimicrob Agents Chemother. 2005;49(12):5092–8.
Pullen J, Stolk LM, Nieman FH, Degraeuwe PL, van Tiel FH, Zimmermann LJ. Population pharmacokinetics and dosing of amoxicillin in (pre)term neonates. Ther Drug Monit. 2006;28(2):226–31.
Pullen J, de Rozario L, Stolk LM, Degraeuwe PL, van Tiel FH, Zimmermann LJ. Population pharmacokinetics and dosing of flucloxacillin in preterm and term neonates. Ther Drug Monit. 2006;28(3):351–8.
García B, Barcia E, Pérez F, Molina IT. Population pharmacokinetics of gentamicin in premature newborns. J Antimicrob Chemother. 2006;58(2):372–9.
Al Z’aabi M, Lanner A, Xiaonian X, Donovan T, Charles B. Application of routine monitoring data for determination of the population pharmacokinetics and enteral bioavailability of phenytoin in neonates and infants with seizures. Ther Drug Monit. 2006;28(6):793–9.
Zuppa AF, Nicolson SC, Adamson PC, Wernovsky G, Mondick JT, Burnham N, et al. Population pharmacokinetics of milrinone in neonates with hypoplastic left heart syndrome undergoing stage I reconstruction. Anesth Analg. 2006;102(4):1062–9.
van Kesteren C, Benders MJ, Groenendaal F, van Bel F, Ververs FF, Rademaker CM. Population pharmacokinetics of allopurinol in full-term neonates with perinatal asphyxia. Ther Drug Monit. 2006;28(3):339–44.
Al Za’abi M, Donovan T, Tudehope D, Woodgate P, Collie LA, Charles B. Orogastric and intravenous indomethacin administration to very premature neonates with patent ductus arteriosus: population pharmacokinetics, absolute bioavailability, and treatment outcome. Ther Drug Monit. 2007;29(6):807–14.
Allegaert K, Peeters MY, Verbesselt R, Tibboel D, Naulaers G, de Hoon JN, et al. Inter-individual variability in propofol pharmacokinetics in preterm and term neonates. Br J Anaesth. 2007;99(6):864–70.
Muller AE, DeJongh J, Bult Y, Goessens WH, Mouton JW, Danhof M, et al. Pharmacokinetics of penicillin G in infants with a gestational age of less than 32 weeks. Antimicrob Agents Chemother. 2007;51(10):3720–5.
Anderson BJ, Allegaert K, Van den Anker JN, Cossey V, Holford NH. Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Br J Clin Pharmacol. 2007;63(1):75–84.
Acosta EP, Brundage RC, King JR, Sánchez PJ, Sood S, Agrawal V, National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group, et al. Ganciclovir population pharmacokinetics in neonates following intravenous administration of ganciclovir and oral administration of a liquid valganciclovir formulation. Clin Pharmacol Ther. 2007;81(6):867–72.
Yukawa E, Akiyama K, Suematsu F, Yukawa M, Minemoto M. Population pharmacokinetic investigation of digoxin in Japanese neonates. J Clin Pharm Ther. 2007;32(4):381–6.
Paradisis M, Jiang X, McLachlan AJ, Evans N, Kluckow M, Osborn D. Population pharmacokinetics and dosing regimen design of milrinone in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007;92(3):F204–9.
Hirt D, Van Overmeire B, Treluyer JM, Langhendries JP, Marguglio A, Eisinger MJ, et al. An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamic study. Br J Clin Pharmacol. 2008;65(5):629–36.
Gregoire N, Desfrere L, Roze JC, Kibleur Y, Koehne P. Population pharmacokinetic analysis of Ibuprofen enantiomers in preterm newborn infants. J Clin Pharmacol. 2008;48(12):1460–8.
Palmer GM, Atkins M, Anderson BJ, Smith KR, Culnane TJ, McNally CM, et al. I.V. acetaminophen pharmacokinetics in neonates after multiple doses. Br J Anaesth. 2008;101(4):523–30.
Lima-Rogel V, Medina-Rojas EL, Del Carmen Milán-Segovia R, Noyola DE, Nieto-Aguirre K, López-Delarosa A, et al. Population pharmacokinetics of cefepime in neonates with severe nosocomial infections. J Clin Pharm Ther. 2008;33(3):295–306.
Bradley JS, Sauberan JB, Ambrose PG, Bhavnani SM, Rasmussen MR, Capparelli EV. Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr Infect Dis J. 2008;27(9):794–9.
Wade KC, Wu D, Kaufman DA, Ward RM, Benjamin DK Jr. Sullivan JE, et al. National Institute of Child Health and Development Pediatric Pharmacology Research Unit Network. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother. 2008;52(11):4043–9.
Charles BG, Townsend SR, Steer PA, Flenady VJ, Gray PH, Shearman A. Caffeine citrate treatment for extremely premature infants with apnea: population pharmacokinetics, absolute bioavailability, and implications for therapeutic drug monitoring. Ther Drug Monit. 2008;30(6):709–16.
Dailly E, Drouineau MH, Gournay V, Rozé JC, Jolliet P. Population pharmacokinetics of domperidone in preterm neonates. Eur J Clin Pharmacol. 2008;64(12):1197–200.
Zuppa AF, Mondick JT, Davis L, Cohen D. Population pharmacokinetics of ketorolac in neonates and young infants. Am J Ther. 2009;16(2):143–6.
Sherwin CM, Svahn S, Van der Linden A, Broadbent RS, Medlicott NJ, Reith DM. Individualised dosing of amikacin in neonates: a pharmacokinetic/pharmacodynamic analysis. Eur J Clin Pharmacol. 2009;65(7):705–13.
Nielsen EI, Sandström M, Honoré PH, Ewald U, Friberg LE. Developmental pharmacokinetics of gentamicin in preterm and term neonates: population modelling of a prospective study. Clin Pharmacokinet. 2009;48(4):253–63.
van den Anker JN, Pokorna P, Kinzig-Schippers M, Martinkova J, de Groot R, Drusano GL, et al. Meropenem pharmacokinetics in the newborn. Antimicrob Agents Chemother. 2009;53(9):3871–9.
Mukherjee A, Dombi T, Wittke B, Lalonde R. Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin Pharmacol Ther. 2009;85(1):56–63.
Hirt D, Urien S, Rey E, Arrivé E, Ekouévi DK, Coffié P, et al. Population pharmacokinetics of emtricitabine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2009;53(3):1067–73.
Hirt D, Urien S, Ekouévi DK, Rey E, Arrivé E, Blanche S, et al. ANRS 12109. Population pharmacokinetics of tenofovir in HIV-1-infected pregnant women and their neonates (ANRS 12109). Clin Pharmacol Ther. 2009;85(2):182–9.
Ahsman MJ, Hanekamp M, Wildschut ED, Tibboel D, Mathot RA. Population pharmacokinetics of midazolam and its metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clin Pharmacokinet. 2010;49(6):407–19.
Marqués-Miñana MR, Saadeddin A, Peris JE. Population pharmacokinetic analysis of vancomycin in neonates: a new proposal of initial dosage guideline. Br J Clin Pharmacol. 2010;70(5):713–20.
Lo YL, van Hasselt JG, Heng SC, Lim CT, Lee TC, Charles BG. Population pharmacokinetics of vancomycin in premature Malaysian neonates: identification of predictors for dosing determination. Antimicrob Agents Chemother. 2010;54(6):2626–32.
Hope WW, Smith PB, Arrieta A, Buell DN, Roy M, Kaibara A, et al. Population pharmacokinetics of micafungin in neonates and young infants. Antimicrob Agents Chemother. 2010;54(6):2633–7.
Blumer J, Rodriguez A, Sánchez PJ, Sallas W, Kaiser G, Hamed K. Single-dose pharmacokinetics of famciclovir in infants and population pharmacokinetic analysis in infants and children. Antimicrob Agents Chemother. 2010;54(5):2032–41.
Xie HG, Cao YJ, Gauda EB, Agthe AG, Hendrix CW, Lee H. Clonidine clearance matures rapidly during the early postnatal period: a population pharmacokinetic analysis in newborns with neonatal abstinence syndrome. J Clin Pharmacol. 2011;51(4):502–11.
Ward RM, Tammara B, Sullivan SE, Stewart DL, Rath N, Meng X, et al. Single-dose, multiple-dose, and population pharmacokinetics of pantoprazole in neonates and preterm infants with a clinical diagnosis of gastroesophageal reflux disease (GERD). Eur J Clin Pharmacol. 2010;66(6):555–61.
Benaboud S, Ekouévi DK, Urien S, Rey E, Arrivé E, Blanche S, TEmAA ANRS 12109 Study Group, et al. Population pharmacokinetics of nevirapine in HIV-1-infected pregnant women and their neonates. Antimicrob Agents Chemother. 2011;55(1):331–7.
Kearns GL, Reed MD. Clinical pharmacokinetics in infants and children: a reappraisal. Clin Pharmacokinet. 1989;17:29–67.
Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr. 2006;165:819–29.
West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284(5420):1677–9.
Anderson BJ, Holford NH. Mechanistic basic of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36.
Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Cole M, Chatelut E, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24(1):67–76.
World Health Organization. WHO model lists of essential medicines. http://whqlibdoc.who.int/hq/2011/a95964_fre.pdf. Accessed 1 Oct 2010.
Jacqz-Aigrain E, Burtin P. Clinical pharmacokinetics of sedatives in neonates. Clin Pharmacokinet. 1996;31(6):423–43.
Rennie JM, Boylan GB. Neonatal seizures and their treatment. Curr Opin Neurol. 2003;6(2):177–81.
Painter MJ, Pippenger C, Wasterlain C, Barmada M, Pitlick W, Carter G, Abern S. Phenobarbital and phenytoin in neonatal seizures: metabolism and tissue distribution. Neurology. 1981;31(9):1107–12.
Tokola RA, Neuvonen PJ. Pharmacokinetics of antiepileptic drugs. Acta Neurol Scand Suppl. 1983;97:17–27.
Acknowledgments
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marsot, A., Boulamery, A., Bruguerolle, B. et al. Population Pharmacokinetic Analysis during the First 2 Years of Life. Clin Pharmacokinet 51, 787–798 (2012). https://doi.org/10.1007/s40262-012-0015-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-012-0015-8